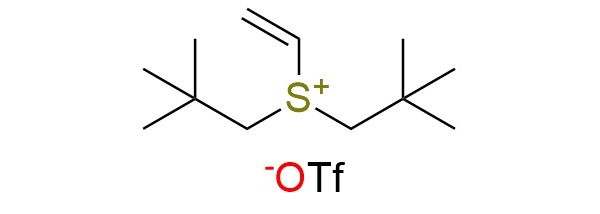
CAS 2956391-81-2, Cat. No EN300-52842679
Reagent for oxetane synthesis
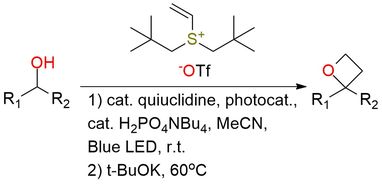
Dineopentylvinylsulfonium triflate is a vinyl sulfonium reagent used for oxetane synthesis from alcohols via photoredox C–H functionalization1,2. The neopentyl groups make it uniquely reactive, enabling radical addition of α-hydroxy radicals followed by 4-exo-tet cyclization with the sulfonium as a leaving group. It shows a broad scope, working with cyclic, acyclic, and heterocyclic alcohols (ethers, esters, sulfones, macrocycles, adamantane, aza-bicycles) and delivers oxetanes in moderate to excellent yields (42–97%). The method offers high site selectivity for α-C–H bonds of alcohols but excludes benzylic, allylic, and propargylic alcohols. This reagent provides a mild, general, and efficient entry to oxetanes.
Synonyms: bis(2,2-dimethylpropyl)(ethenyl)sulfanium trifluoromethanesulfonate; bis(neopentyl)-vinyl-sulfonium trifluoromethanesulfonate
Selected publications
-
Oxetanes: Recent Advances in Synthesis, Reactivity, and Medicinal Chemistry.
Bull, J. A.; Croft, R. A.; Davis, O. A.; Doran, R.; Morgan, K. F. Chemical Reviews 2016, 12150–12233. DOI: 10.1021/acs.chemrev.6b00274
-
Oxetane Synthesis via Alcohol C-H Functionalization.
Paul, S.; Filippini, D.; Ficarra, F.; Melnychenko, H.; Janot, C.; Silvi, M. J Am Chem Soc 2023, 145 (29), 15688–15694. DOI: 10.1021/jacs.3c04891