CAS 2166-14-5, Cat. No EN300-211397
Versatile building block in Retro-Diels-Alder reactions
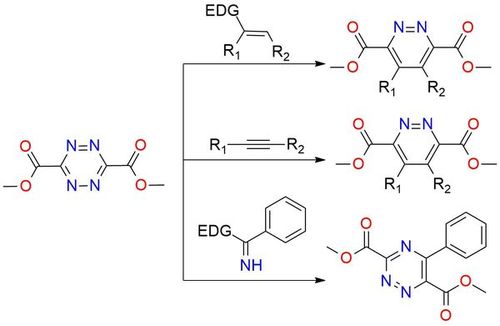
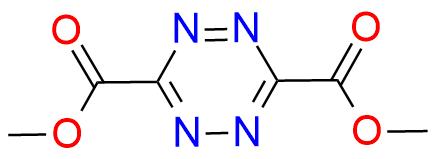
Dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate is a highly versatile heteroaromatic azadiene that has garnered considerable attention for its exceptional reactivity and its ability to engage in retro-Diels-Alder reaction with a wide range of dienophiles and heterodienophiles1. This compound enables the synthesis of substituted 1,2-diazines, 1,2,4-triazines, pyrroles, pyridines, indolines, and related condensed heterocycles through cycloaddition reactions that have an operationally simple procedure2,3. The mildness of the reaction conditions and predictable positional selectivity, high level of functional group compatibility make this heteroaromatic azadiene perfect as a reagent for pharmaceutically important targets1,2,4.
Synonyms: 3,6-dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate
Selected publication
-
The Retro–Diels–Alder Reaction Part II. Dienophiles with One or More Heteroatom.
Rickborn B. Organic Reactions 1998, 223–629. DOI: 10.1002/0471264180.or053.02
-
Dimethyl 1,2,4,5-Tetrazine-3,6-Dicarboxylate.
Boger D.; Zhang M.; Haider N. Encyclopedia of Reagents for Organic Synthesis 2006. DOI: 10.1002/047084289X.rd389.pub2
-
Preparation and diels-alder reaction of a reactive, electron-deficient heterocyclic azadiene: dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate. 1,2-diazine (dimethyl 4-phenyl-1,2-diazine-3,6-dicarboxylate) and pyrrole (dimethyl 3-phenylpyrrole-2,5-dicarboxylate) introduction.
Organic Syntheses 1992, 70, 79. DOI: 10.15227/orgsyn.070.0079
-
Detailed, Convenient Preparation of Dimethyl 1,2,4,5-Tetrazine-3,6-Dicarboxylate.
Boger D.; Coleman R.; Panek J.; Huber F.; Sauer J. J Org Chem 1985, 50 (25), 5377–5379. DOI: 10.1021/jo00225a076