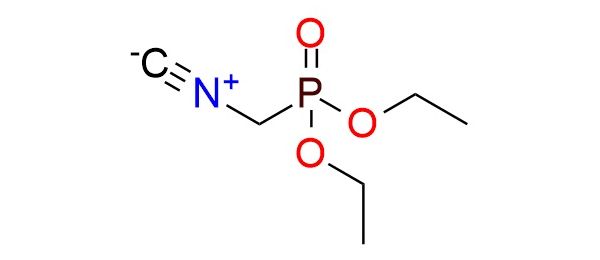
CAS 41003-94-5, Cat. No EN300-343415
Reagent for HWE reaction and heterocycles synthesis
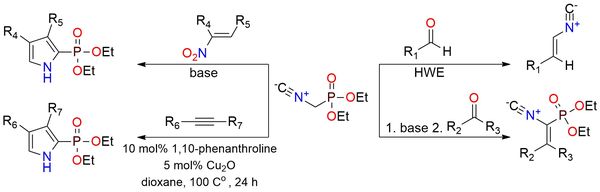
Diethyl (isocyanomethyl)phosphonate is a versatile isocyanide-bearing phosphonate reagent used to extend aldehydes and ketones by one carbon and to build a wide variety of heterocycles and functionalized intermediates1. It behaves as an anionic Horner–Wadsworth–Emmons (HWE) olefination reagent, producing vinyl isocyanides from carbonyl compounds. Under base-promoted conditions, it undergoes Knoevenagel-type condensations to form α-isocyano vinyl phosphonates, which can be transformed into progesterone derivatives via reduction and rearrangement. This reagent also participates in oxazoline formation, oxazole synthesis, imidazole assembly, and pyrrole-forming reactions with nitroalkenes and alkynes. The reagent is a stable, colorless liquid that is distillable but requires storage at a low temperature.
Synonyms: 1-[ethoxy(isocyanomethyl)phosphoryl]oxyethane; (diethoxycarbonyl)methanisocyanide; diethyl(isocyanomethyl)phosphonate; diethyl isocyanomethylphosphonate
Selected publication
-
Diethyl Isocyanomethylphosphonate.
van Leusen, A. M.; van Leusen, D.; Mitamura, T.; Ogawa, A. Encyclopedia of Reagents for Organic Synthesis 2009. DOI: 10.1002/047084289X.rd197.pub2