CAS 919-19-7, Cat. No EN300-257056
Reagent acyl-anion equivalent
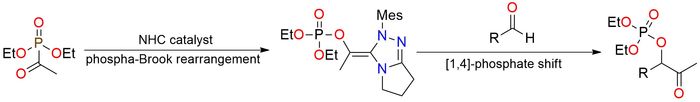
Diethyl acetylphosphonate is a reagent that functions as a masked acyl-anion equivalent under N-heterocyclic carbene (NHC) catalysis. It serves as the key substrate enabling the tandem [1,2]-phospha-Brook/[1,4]-phosphate rearrangement, a transformation that converts aldehydes into α-ketophosphates in high yields1. The methodology shows a broad scope of substrates, reacting efficiently with electron-rich and electron-poor aromatic aldehydes as well as challenging aliphatic aldehydes. The process is cyanide-free, mild, and highly chemoselective, offering a practical route to synthetically valuable α-ketophosphates. Overall, diethyl acetylphosphonate is a stable, versatile, and highly effective acyl phosphonate reagent, uniquely suited for NHC-driven acyl-anion chemistry and tandem rearrangement processes.
Synonyms: O,O-diethyl acetylphosphonate; 1-diethoxyphosphorylethanone; 1-(diethoxyphosphinyl)ethenone; diethyl (1-oxoethyl)phosphonate; acetylphosphonic acid diethyl ester
Selected publication
-
Carbene-Catalyzed Tandem [1,2]-Phospha-Brook/[1,4]-Phosphate Rearrangement: Access to α-Ketophosphates via Controlled Cross-Acyloin Condensation.
Verma, R. S.; Pandey, C. B.; Kumar, S.; Tiwari, B. Journal of Organic Chemistry 2018, 83 (16), 9478–9483. DOI: 10.1021/acs.joc.8b01172

