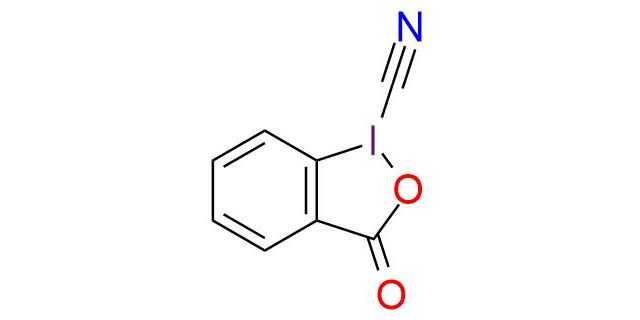
CAS 172876-96-9, Cat. No EN300-7867959
Reagent for radical cyanation
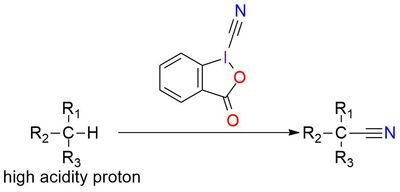
3-Oxo-1,2-benziodoxole-1(3H)-carbonitrile is part of the stable benziodoxole “atom-transfer” reagents family. In very mild conditions (for example, heating) it releases a cyano radical that can undergo various reactions of cyanation1. The reagent is stable solid, soluble in DMSO, and insoluble in ether and nonpolar organic solvents2. In cases with low acidity of proton for cyanation, a base can be used for the deprotonation of substrate. Also, chiral organocatalysts can be implied for enantioselectivity of the reaction and such examples are reported, for example, in the reaction of asymmetric cyanation of β-keto esters1,2. The reagent usage is not limited to CH bond cyanation, it works for NH and SH bond, but CH cyanation makes it special1.
Synonyms: 3-Oxo-1,2-benziodoxole-1(3H)-carbonitrile
Selected publication
-
Recent Progress in Synthetic Applications of Cyclic Hypervalent Iodine(III) Reagents.
Yoshimura A.; Saito A.; Zhdankin V. Adv Synth Catal 2023, 365 (16), 2653–2675. DOI: 10.1002/adsc.202300275
-
1,2-Benziodoxol-3(1H)-One Derivative.
Zhdankin V. Encyclopedia of Reagents for Organic Synthesis 2005. DOI: 10.1002/047084289X.rn00595