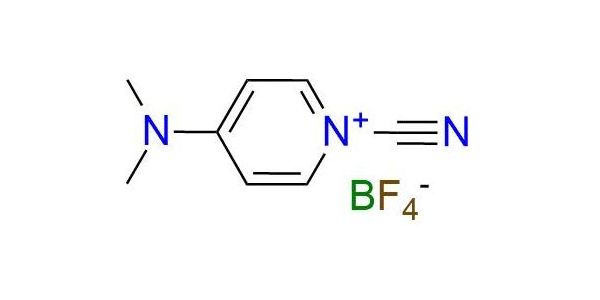
CAS 59016-56-7, Cat. No EN300-6731863
Reagent for cyanation
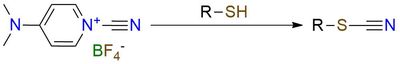
This reagent is capable of selective cyanation of thiols. It is a bench-stable solid, soluble in most common organic solvents, both protic and aprotic. The reaction occurs with many nucleophiles but specifically prioritizes thiols in the presence of hydroxy or amino group1,2. This reagent finds its uses in selective cyanation of the peptides, which is often completed without any problem and with good yield2. Also, this reagent is known to be used for cyanation of Grignard reagents3.
Synonyms: borate(1-), tetrafluoro-, 1-cyano-4-(dimethylamino)pyridinium (ZCI); pyridinium, 1-cyano-4-(dimethylamino)-, tetrafluoroborate(1-) (9CI); 1-cyano-4-dimethylammoniumpyridinium tetrafluoroborate; 1-cyano-N,N-dimethyl-1,4-dihydropyridin-4-iminium; tetrafluoroboranuide
Selected publication
-
A Novel Methodology for Assignment of Disulfide Bond Pairings in Proteins.
Wu J.; Watson J. Protein Science 1997, 6 (2), 391–398. DOI: 10.1002/pro.5560060215
-
1-Cyano-4-Dimethylamino-Pyridinium Salts: New Water-Soluble Reagents for the Cyanylation of Protein Sulphydryl Groups.
Wakselman M.; Guibé-Jampel E.; Raoult A.; Busse W. J. Chem. Soc., Chem. Commun. 1976, 1, 21–22. DOI: 10.1039/C39760000021
-
Convenient Synthesis of Benzonitriles via Electrophilic Cyanation with N‐Cyanobenzimidazole.
Anbarasan P.; Neumann H.; Beller M. Chemistry – A European Journal 2010, 16 (16), 4725–4728. DOI: 10.1002/chem.201000086