CAS 105850-89-3, Cat. No EN300-253475
Thiocarbonyl ylide precursor
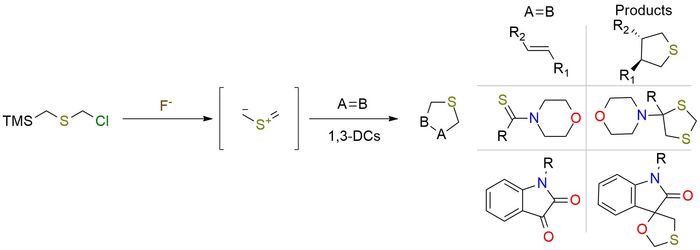
Chloromethyl (trimethylsilyl)methyl sulfide is a valuable source of thiocarbonyl ylide, which exhibits rapid reactivity in 1-3 dipolar cycloadditions [3+2] or [3+3], leading to the formation of S-heterocycles1-3. The formation of intermediate occurs in the presence of F- anion from salts (CsF, N+Me4F- for example). The versatility of thiocarbonyl ylide is evident in its reaction with a diverse range of substrates1. It demonstrates compatibility with aliphatic aldehydes, aryl isothiocyanates, and activated four- and five-membered alkenes1,3. By harnessing the unique reactivity of chloromethyl (trimethylsilyl)methyl sulfide and its resulting thiocarbonyl ylide, chemists can access a wide array of S-heterocycles and engage in diverse synthetic transformations.
Synonyms: chloromethyl (trimethylsilyl)methylsulfide; chloromethylsulfanylmethyl(trimethyl)silane; (((chloromethyl)thio)methyl)trimethylsilane
Selected publication
-
Synthesis of 4-Polyfluoroalkyl-1,3-Dithiolanes via [3+2] Cycloaddition of Thiocarbonyl Ylide to Polyfluoroalkanethioamides.
Mykhaylychenko S.; Markitanov Y.; Rudenko T.; Rusanov E.; Shermolovich Y. Chem Heterocycl Compd 2019, 55 (2), 189–192. DOI: 10.1007/s10593-019-02438-0
-
Chloromethyl Trimethylsilylmethyl Sulphide as a Parent Thiocarbonyl Ylide Synthon. A Simple Synthesis of Dihydro- and Tetrahydro-Thiophenes.
Hosomi A.; Matsuyama Y.; Sakurai H. J Chem Soc Chem Commun 1986, 14, 1073. DOI: 10.1039/c39860001073
-
Construction of Multifunctional Modules for Drug Discovery: Synthesis of Novel Thia/Oxa-Azaspiro[3.4]Octanes.
Li D.; Rogers-Evans M.; Carreira E. Org Lett 2013, 15 (18), 4766–4769. DOI: 10.1021/ol402127b