CAS 7552-55-8, Cat. No EN300-727934
Reagent for SuFEx deoxyazidation and amide coupling
![1-((3,5-Difluorophenyl)sulfonyl)bicyclo[1.1.0]butane](/images/Reagents/EN300-727934_Scheme.jpg)
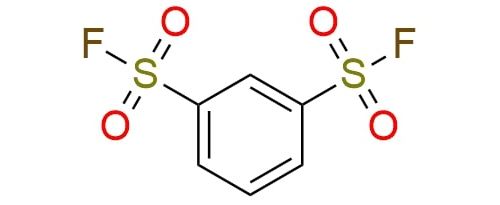
Benzene-1,3-disulfonyl fluoride (BDSF) is a SuFEx reagent used in deoxyazidation reactions for converting hydroxy group into azide1. With synergistic interaction with azidotrimethylsilane (TMSN3) under mild conditions, the reagent transforms primary and secondary alcohols into azides. BDSF activates alcohols by forming sulfonate intermediates with high yields. It is suitable for a wide range of substrates, including complex molecules containing esters, amides, and heterocycles. Although BDSF requires higher reagent loading than benzene-1,3,5-trisulfonyl fluoride (BTSF), it remains efficient in functionalizing diverse compounds. Besides BDSF can serve as a coupling agent in amide bond formation2. It is efficient in synthesizing sterically hindered and electron-deficient secondary and tertiary amides via acyl fluoride intermediates. The scope of substrates in SuFEx-mediated amide synthesis includes a wide range of amines (e.g. benzylamines, anilines, and aliphatic amines) and carboxylic acids. The protocol handles sterically hindered substrates like adamantane carboxylic acid and arylacetic acids, yielding products in moderate to excellent yields.
Synonyms: 1,3-benzenedisulfonyl difluoride; benzene-1,3-disulfonyl fluoride; benzene-1,3-disulfonyl difluoride; 1,3-benzenedisulfonyl fluoride; 1,3-benzenedisulfonylfluoride; BDSF
Selected publications
-
Benzene‐1,3‐disulfonyl Fluoride and Benzene‐1,3,5‐trisulfonyl Fluoride: Low‐Cost, Stable, and Selective Reagents for SuFEx‐Driven Deoxyazidation.
Vishwakarma D.; Moses J. Adv Synth Catal 2024. DOI: 10.1002/adsc.202400680
-
Sulfur–Fluoride Exchange (SuFEx)‐Mediated Synthesis of Sterically Hindered and Electron‐Deficient Secondary and Tertiary Amides via Acyl Fluoride Intermediates.
Smedley C.; Barrow A.; Spiteri C.; Giel M.; Sharma P.; Moses J. Chemistry – A European Journal 2017, 23 (42), 9990–9995. DOI: 10.1002/chem.201701552