CAS 1401714-42-8, Cat. No EN300-23000638
Reagent for fluoroalkylation
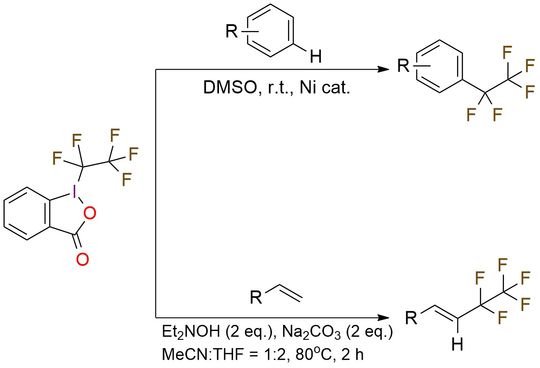
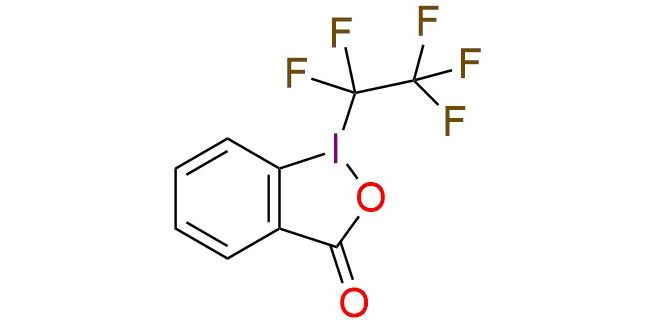
1-(1,1,2,2,2-Pentafluoroethyl)-1,2-benziodoxol-3(1H)-one (acid C2F5–Togni reagent) is a hypervalent iodine(III) compound structurally related to Togni’s trifluoromethylating reagents. It serves as a convenient and stable source of the pentafluoroethyl (C2F5) radical. In ligand-free nickel catalysis1, it enables direct C–H pentafluoroethylation of arenes and heteroarenes. Electron-rich substrates such as trimethoxybenzene and pyrimidines give high yields of 75–97%, while pyrroles and indoles react in moderate to good yields of 30–73%. The reaction shows good tolerance of halogens and Boc-protected substrates, with regioselectivity governed by electronic and steric effects. In the hydroxylamine-mediated protocol2, the reagent is used for radical addition–elimination on alkenes, producing vinyl–C2F5 products. A broad range of substrates, including styrenes, disubstituted alkenes, 1,2-dihydronaphthalenes, and 1,3-dienes, give yields of 68–99%. This method delivers excellent E/Z stereoselectivity, typically greater than 20:1, and tolerates a wide array of functional groups, including halides in all positions, nitro groups, esters, dimethoxy, and dimethyl substituents, and heteroaryl systems.
Synonyms: 1-(1,1,2,2,2-pentafluoroethyl)-1,2-benziodoxol-3(1H)-one; 1,2-benziodoxol-3(1H)-one, 1-(1,1,2,2,2-pentafluoroethyl)-; 1-(1,1,2,2,2-pentafluoroethyl)-3H-1lambda3,2-benziodaoxol-3-one; C2F5–Togni reagent
Selected publications
-
Ligand-Free Nickel Catalyzed Perfluoroalkylation of Arenes and Heteroarenes.
Deolka, S.; Govindarajan, R.; Vasylevskyi, S.; Roy, M. C.; Khusnutdinova, J. R.; Khaskin, E. Chem Sci 2022, 509. DOI: 10.1039/d2sc03879j
-
Hydroxylamine-Mediated C(Sp2)−H Trifluoromethylation of Terminal Alkenes.
Zhao, X.; Zhong, B.; Dong, L.; Zhang, Y. S.; Luo, H. T.; Yang, J. D.; Cheng, J. P. Chemistry - A European Journal 2024, 30 (33). DOI: 10.1002/chem.202400995