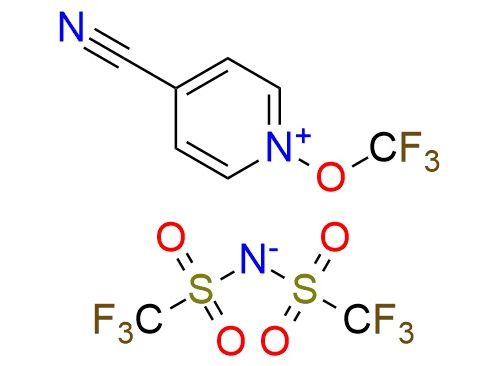
CAS 2242839-02-5, Cat. No EN300-26938806
Reagent for trifluoromethoxylation
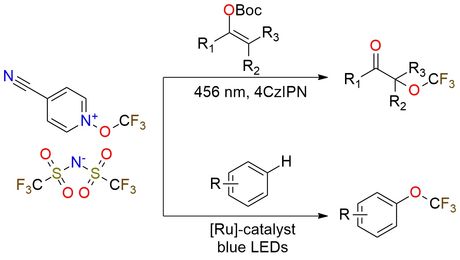
4-Cyano-N-trifluoromethoxypyridinium triflimide is a versatile reagent for radical trifluoromethoxylation, enabling the direct introduction of the trifluoromethoxy group (-OCF3) under visible-light photoredox catalysis. It is used for regioselective C–H functionalization of arenes1 and α-trifluoromethoxylation of ketones2, offering high chemoselectivity and efficiency. The reagent is particularly valuable for functionalizing bioactive molecules, including natural products and pharmaceutical precursors. It is bench-stable and thermally robust up to 240°C, can be stored under ambient conditions, and is compatible with a wide range of functional groups.
Synonyms: 4-cyano-N-trifluoromethoxypyridinium triflimide; 4-cyano-1-(trifluoromethoxy)pyridinium bis(trifluoromethanesulfonimide); 4-cyano-1-(trifluoromethoxy)pyridin-1-ium bis((trifluoromethyl)sulfonyl)amide; bis(trifluoromethylsulfonyl)azanide;1-(trifluoromethoxy)pyridin-1-ium-4-carbonitrile; N-trifluoromethoxy-4-cyanopyridinium triflimide
Selected publications
-
Radical Trifluoromethoxylation of Arenes Triggered by a Visible‐Light‐Mediated N−O Bond Redox Fragmentation.
Jelier B.; Tripet P.; Pietrasiak E.; Franzoni I.; Jeschke G.; Togni A. Angewandte Chemie International Edition 2018, 57 (42), 13784–13789. DOI: 10.1002/anie.201806296
-
Radical α-Trifluoromethoxylation of Ketones under Batch and Flow Conditions by Means of Organic Photoredox Catalysis.
Duhail T.; Bortolato T.; Mateos J.; Anselmi E.; Jelier B.; Togni A.; Magnier E.; Dagousset G.; Dell’Amico L. Org Lett 2021, 23 (18), 7088–7093. DOI: 10.1021/acs.orglett.1c02494