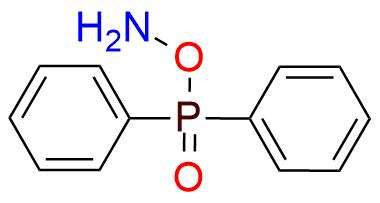
CAS 72804-96-7, Cat. No EN300-101508
Reagent for electrophilic amination and N-deletion in amines
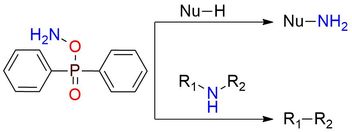
DPPH (O-diphenylphosphinyl hydroxylamine) is an electrophilic amination reagent that occupies a unique niche in organic synthesis. It is a solid, bench-stabile for a year compound with very low solubility in aprotic organic solvents1. This reagent rapidly reacts with the NH group in imidazoles, xanthines, indoles, and carbazoles. The usage of DPPH is a reliable method for N-amination of many primary and secondary amines2,3 that starts with deprotonation (e.g. with NaH) followed by further treatment with the reagent. Stabilized carbanions or Grignard reagents can serve as substrates for reaction as well1,2. The newly developed methodology allows nitrogen deletion with the DPPH from secondary amines4. This reaction demonstrates high functional tolerance and can be applied to α-primary, α-secondary, and α-tertary alkylamines.
Synonyms: azanyl P,P-diphenylphosphinate (ACI); hydroxylamine, O-(diphenylphosphinyl)- (9CI); (aminooxy)diphenylphosphine oxide; O-(diphenylphosphoryl)hydroxylamine; O-diphenylphosphinylhydroxylamine
Selected publication
-
O-(Diphenylphosphinyl)Hydroxylamine.
Boche G.; Belani J. D. Encyclopedia of Reagents for Organic Synthesis 2009. DOI: 10.1002/047084289X.rd432.pub2
-
(Diphenylphosphinyl)Hydroxylamine: A New Reagent for Electrophilic-Amination.
Colvin E. W.; Kirby G. W.; Wilson A. C. Tetrahedron Lett 1982, 23 (37), 3835–3836. DOI: 10.1016/S0040-4039(00)87720-5
-
Scalable Preparation of O-(Diphenylphosphinyl) Hydroxylamine (DPPH).
Tamas T. Organic Syntheses 2020, 97, 54–65. DOI: 10.15227/orgsyn.097.0054
-
C(sp3)–C(sp3) Bond Formation through Nitrogen Deletion of Secondary Amines Using O-Diphenylphosphinylhydroxylamine.
Guo T.; Li J.; Cui Z.; Wang Z.; Lu H. Nature Synthesis 2024. DOI: 10.1038/s44160-024-00559-9