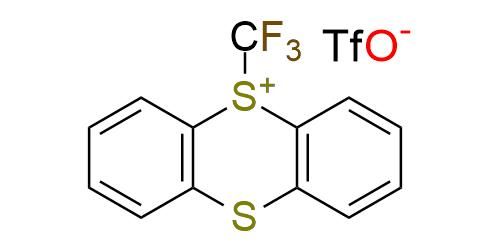
CAS 2648079-79-0, Cat. No EN300-43352843
Reagent for trifluoromethylation
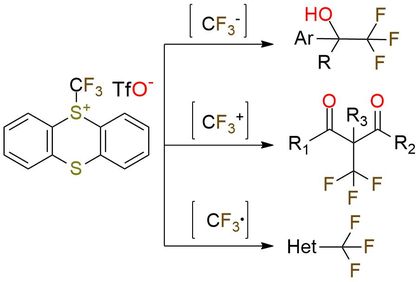
S-(Trifluoromethyl)thianthrenium triflate (TT-CF3+OTf-) is a trifluoromethylating reagent that can produce electrophilic, nucleophilic, or radical CF3 moiety depending on conditions1. The compound works as a universal trifluoromethylating reagent that covers a wide scope of substrates. It converts the alcohol group into fluorides, carbonyl to CF2, and carboxylic into the fluoracyl group. The substrate scope of the trifluoromethylation and the functional groups tolerance are wide: esters, ethers, nitrogen heterocycles, and amides, as well as complex small molecules, are among2,3. Moreover, styrene derivatives, which often engage in unproductive polymerization, isomerization, and dimerization, are tolerated in the transformation1.
Synonyms: TT-CF3+OTf-; 5-(trifluoromethyl)-5H-thianthren-5-ium trifluoromethanesulfonate; S-(trifluoromethyl)thianthrenium triflate
Selected publications
-
Trifluoromethyl Thianthrenium Triflate: A Readily Available Trifluoromethylating Reagent with Formal CF3+, CF3•, and CF3– Reactivity.
Jia H.; Häring A.; Berger F.; Zhang L.; Ritter T. J Am Chem Soc 2021, 143 (20), 7623–7628. DOI: 10.1021/jacs.1c02606
-
Visible-Light-Induced Photocatalyst-Free Radical Trifluoromethylation.
Li Y.; Liang X.; Niu K.; Gu J.; Liu F.; Xia Q.; Wang Q.; Zhang W. Org Lett 2022, 24 (32), 5918–5923. DOI: 10.1021/acs.orglett.2c02150
-
Recent Advances in the Trifluoromethylation Methodology and New CF3-Containing Drugs.
Zhu W.; Wang J.; Wang S.; Gu Z.; Aceña J.; Izawa K.; Liu H.; Soloshonok V. J Fluor Chem 2014, 167, 37–54. DOI: 10.1016/j.jfluchem.2014.06.026