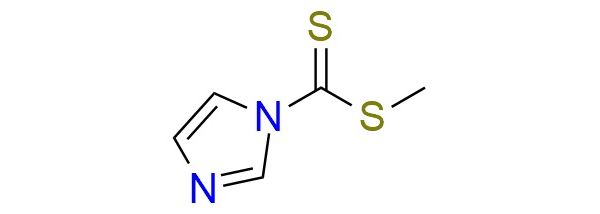
CAS 74734-11-5, Cat. No EN300-7247412
Reagent for thiocarbonyl transfer
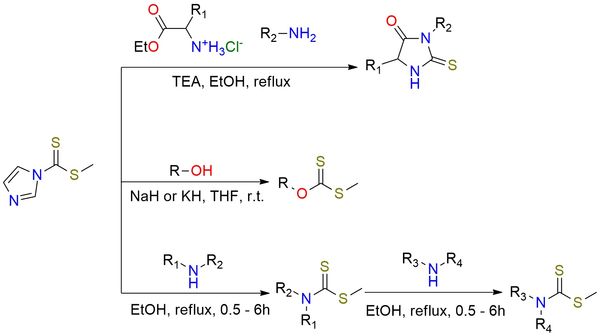
1-(Methyldithiocarbonyl) imidazole is a yellow crystalline solid, soluble in most common organic solvents, and stable under cool and dry conditions1,2. The reagent is widely used because it efficiently transfers a thiocarbonyl group. It forms dithiocarbonates (xanthates) from alcohols in excellent yields (>95%), and dithiocarbamates from amines or anilines, with even higher efficiency when its methyl iodide adduct is used. It also produces thioureas and heterocyclic thiones from diamines or amino alcohols, giving both symmetrical and unsymmetrical products in good yields. The key application is in the synthesis of 2-thiohydantoins3: in three-component couplings with amino esters and amines, it provides 3,5- and 1,3,5-substituted or fused thiohydantoins in yields of 87–97%, often with good stereochemical integrity. In summary, it is a practical, safe, and versatile alternative to the traditional CS2/MeI system for thiocarbonyl transfer, with broad use in the synthesis of sulfur-containing heterocycles and thioureas.
Synonyms: 1H-Imidazole-1-carbodithioic acid, methyl ester; methyl 1H-imidazole-1-carbodithioate
Selected publications
-
1H-Imidazole-1-Carbodithioic Acid, Methyl Ester.
Brown, M. K. Encyclopedia of Reagents for Organic Synthesis 2010. DOI: 10.1002/047084289X.rn01125
-
1-(Methyldithiocarbonyl)Imidazole: A Reagent of S -Methyldithiocarbonylation.
Sun, W.; Hu, J.; Shi, Y. Synlett 1997, 1997 (11), 1279–1280. DOI: 10.1055/s-1997-1019
-
1-(Methyldithiocarbonyl)Imidazole as Thiocarbonyl Transfer Reagent: A Facile One-Pot Three-Component Synthesis of 3,5- and 1,3,5-Substituted-2- Thiohydantoins.
Sundaram, G. S. M.; Venkatesh, C.; Ila, H.; Junjappa, H. Synlett 2007, 2, 251–254. DOI: 10.1055/s-2007-967987