CAS 103020-98-0, Cat. No EN300-249400
Reagent for SuFEx chemistry
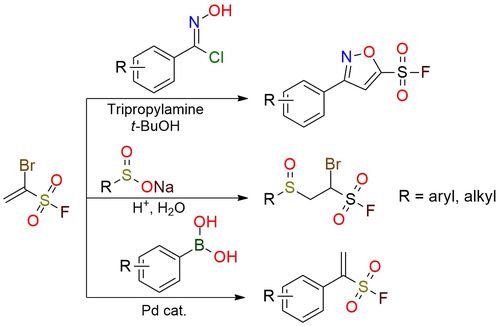
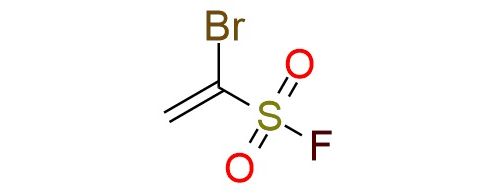
1-Bromoethene-1-sulfonyl fluoride is a versatile and highly reactive SuFEx (Sulfur Fluoride Exchange) clickable reagent with three orthogonal functional groups: a vinyl moiety, a bromide group, and a sulfonyl fluoride functionality1. Its applications span across click chemistry, regioselective cycloadditions, and Michael additions2. Notably, it enables the synthesis of 5-sulfonylfluoro isoxazoles and α-(hetero)aryl ethenesulfonyl fluorides through [3+2] cycloadditions and Suzuki coupling, respectively3. The reagent's scope includes the formation of isoxazole-based pharmacophores and sulfonyl fluoride derivatives, which are valuable in medicinal chemistry, chemical biology, and polymer synthesis. Bromoethene-1-sulfonyl fluoride is a colorless, moisture-sensitive liquid that requires careful handling under dry and inert conditions. It demonstrates excellent reactivity but decomposes in the presence of bases or under high-temperature conditions.
Synonyms: 1-bromoethene-sulfonyl fluoride; ethenesulfonyl fluoride, 1-bromo-; 1-Br-ESF
Selected publications
-
1-Bromoethene-1-Sulfonyl Fluoride (1-Br-ESF), a New SuFEx Clickable Reagent, and Its Application for Regioselective Construction of 5-Sulfonylfluoro Isoxazoles.
Leng J.; Qin H. Chemical Communications 2018, 54 (35), 4477–4480. DOI: 10.1039/c8cc00986d
-
Green and Efficient Synthesis of Pure β-Sulfonyl Aliphatic Sulfonyl Fluorides through Simple Filtration in Aqueous Media.
Feng K.; Wang J.; Zhang W.; Qin H. Org Biomol Chem 2023, 21 (24), 4967–4971. DOI: 10.1039/d3ob00669g
-
Construction of α-(Hetero)Aryl Ethenesulfonyl Fluorides for SuFEx Click Chemistry.
Leng J.; Alharbi N.; Qin H. European J Org Chem 2019, 2019 (35), 6101–6105. DOI: 10.1002/ejoc.201901106