CAS 52509-14-5, Cat. No EN300-170136
Reagent for Wittig reaction
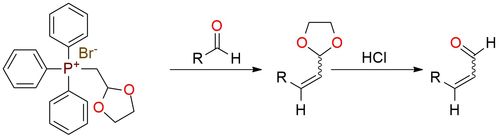
(1,3-Dioxolan-2-ylmethyl)triphenylphosphonium bromide is a phosphonium salt used in organic synthesis, particularly for Wittig reaction1. It reacts with aldehydes to form α,β-unsaturated aldehydes through alkenation. Reactions of the ylide with ketones generally result in poor yields. The stereochemistry of the alkenes formed is highly dependent on the choice of base and solvent. The reagent is commonly used for the two-carbon homologation of aldehydes and can produce both (E)- and (Z)-alkenes, with the stereoselectivity adjustable by altering reaction conditions. (E)-Alkenes are favored when using lithium methoxide or potassium carbonate in DMF. (Z)-Alkenes are preferred with DMSO or THF, with selectivity ratios as high as 8:1 for the Z-isomer.
Synonyms: ((1,3-dioxolan-2-yl)methyl)triphenylphosphonium bromide; [(1,3-dioxolan-2-yl)methyl]triphenylphosphonium bromide; (1,3-Dioxolan-2-yl)methyltriphenylphosphonium bromide; 1,3-dioxolan-2-ylmethyl(triphenyl)phosphanium bromide; 1,3-dioxolan-2-ylmethyl(triphenyl)phosphonium bromide; (1,3-dioxolan-2-ylmethyl)triphenyl-phosphonium bromide
Selected publication
-
(1,3-Dioxolan-2-Ylmethyl)Triphenylphosphonium Bromide.
McComsey D.; Maryanoff B.; Reid M.; Taylor R. Encyclopedia of Reagents for Organic Synthesis 2006. DOI: 10.1002/047084289X.rd406.pub2