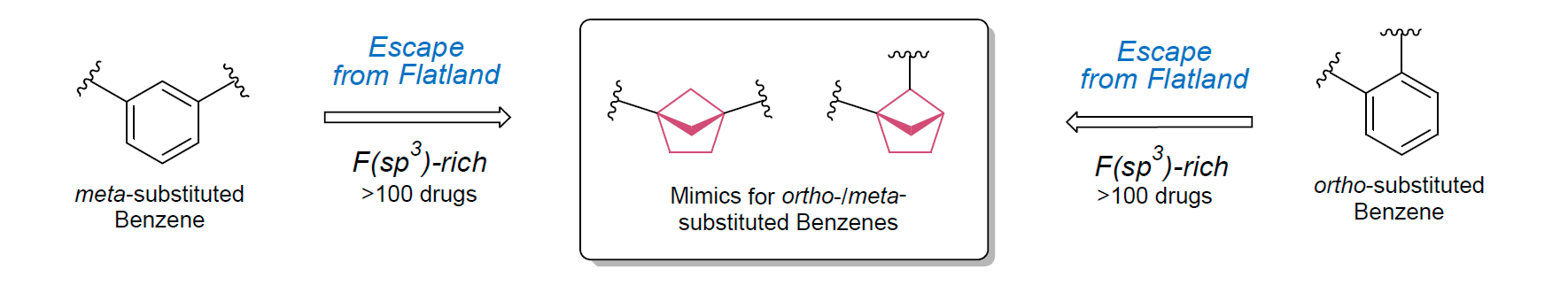
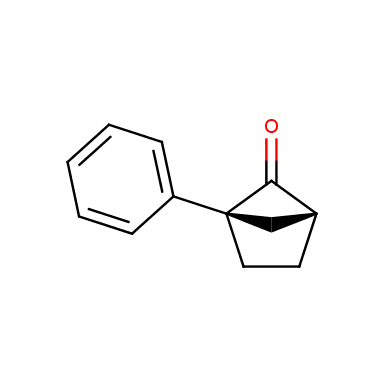
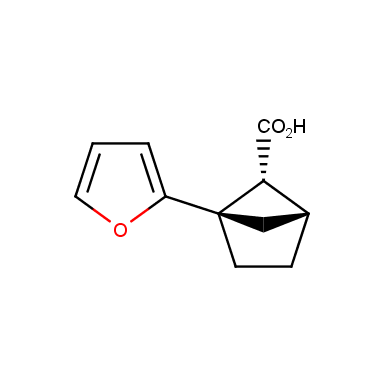
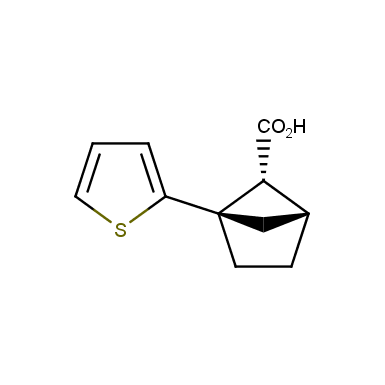
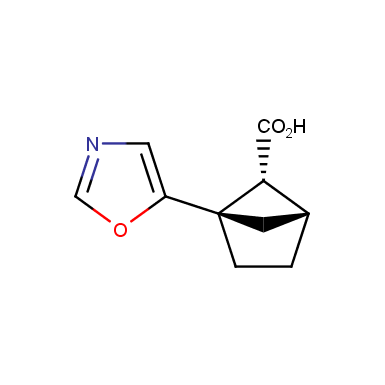
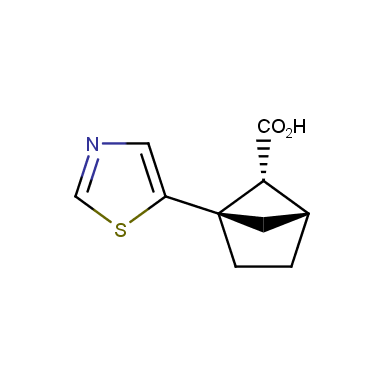
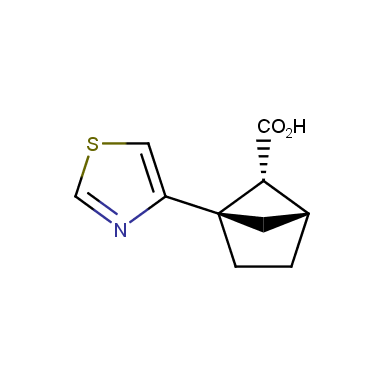
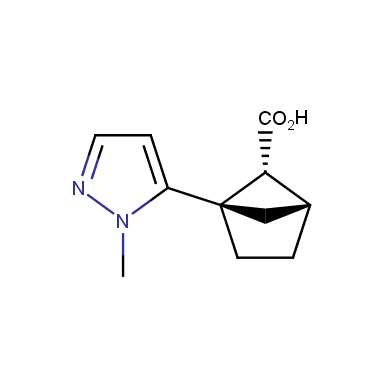
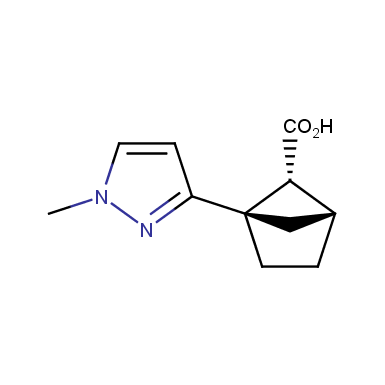
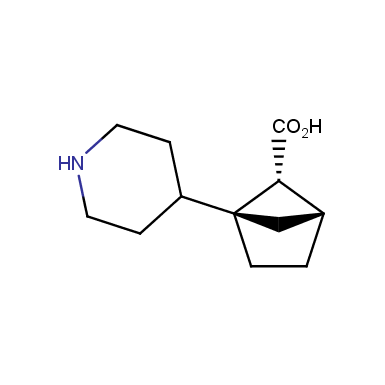
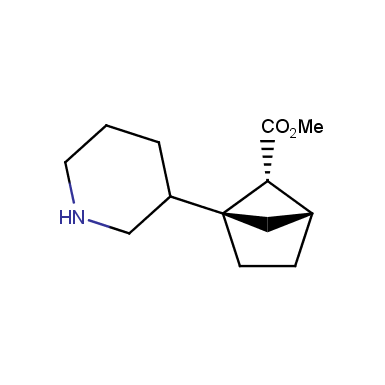
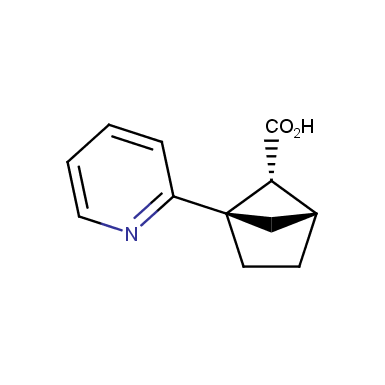
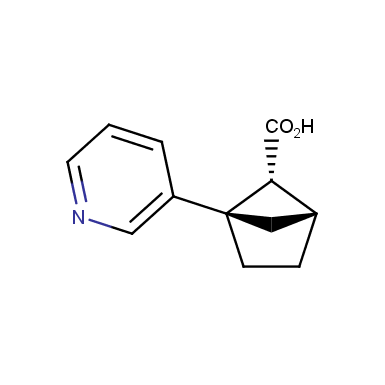
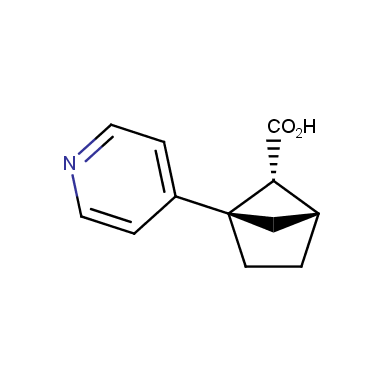
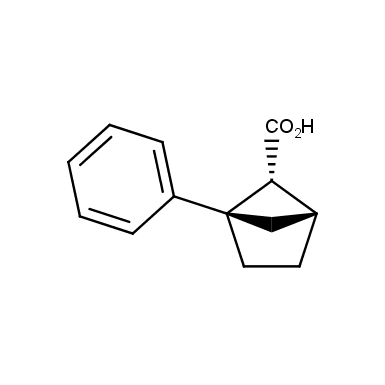
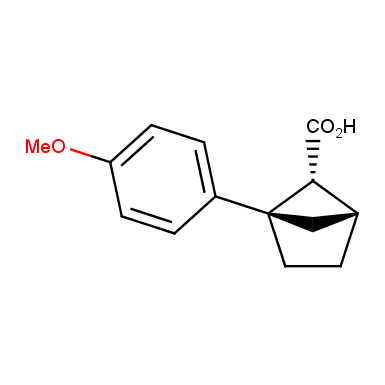
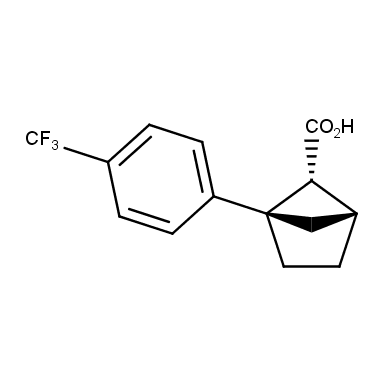
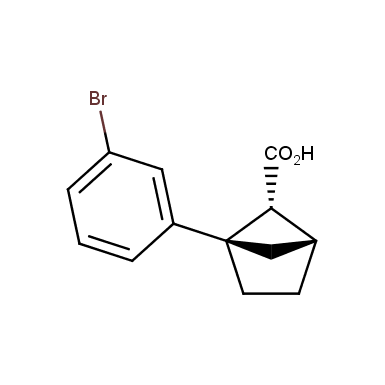
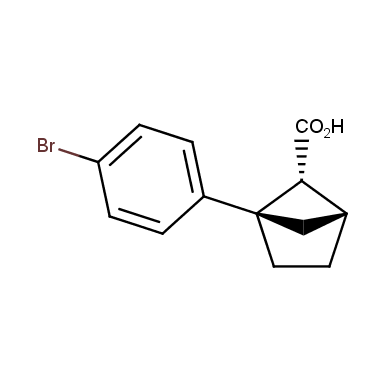
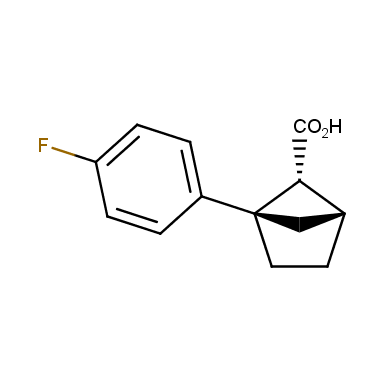
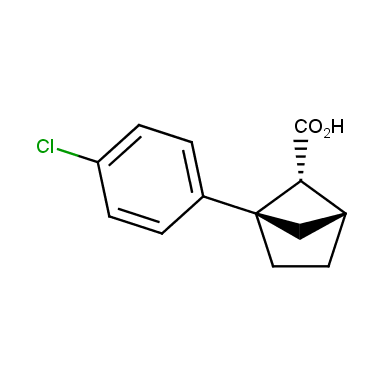
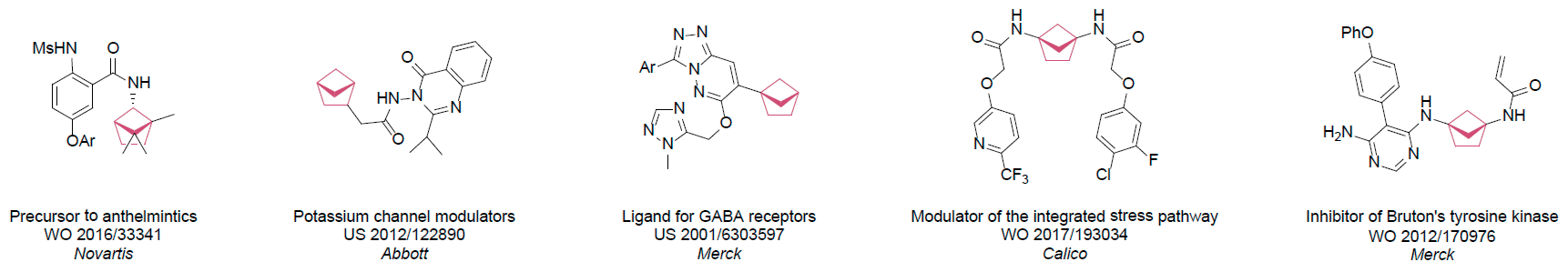
The fragment of benzene comprises to the structure of more than 500 FDA-approved drugs. In 2012, Stepan and coworkers showed that bicyclo[1.1.1]pentane skeleton could act as a saturated “nonclassical phenyl ring bioisostere”. Adding one carbon atom gives the closest homologue – bicyclo[2.1.1]hexane. The lack of the practical synthetic approaches restricts the common use of bicyclo[2.1.1]hexanes in chemistry. Herein we have designed and synthesized a library of saturated mimics of the ortho- and meta-benzene ring for drug design.
Design