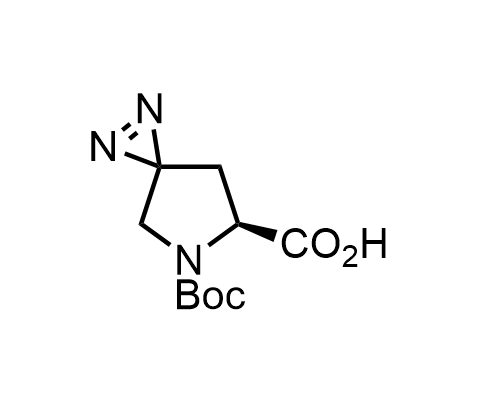
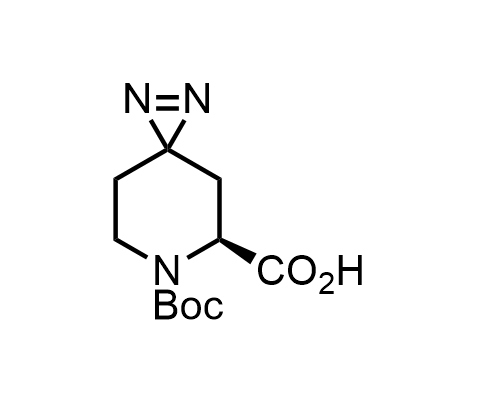
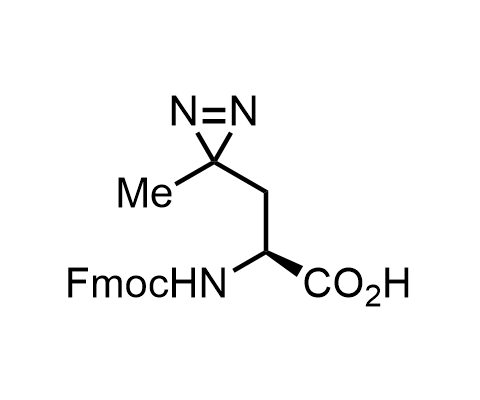
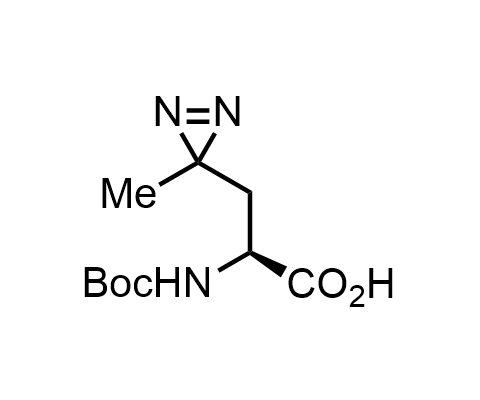
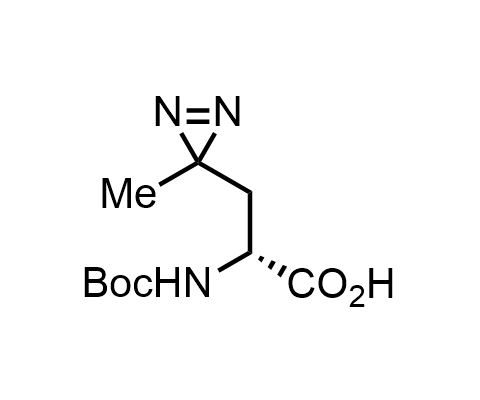
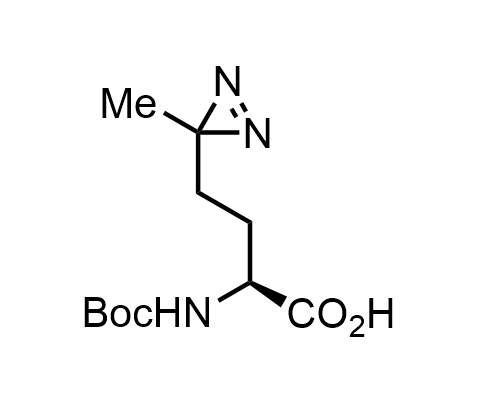
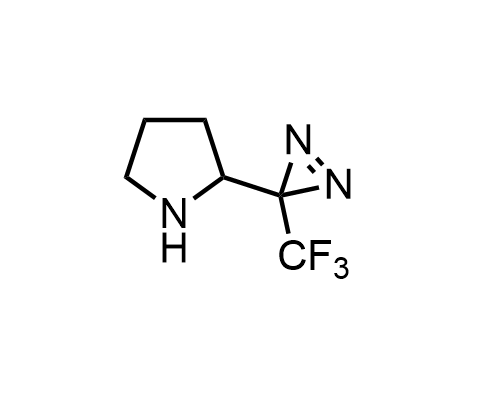
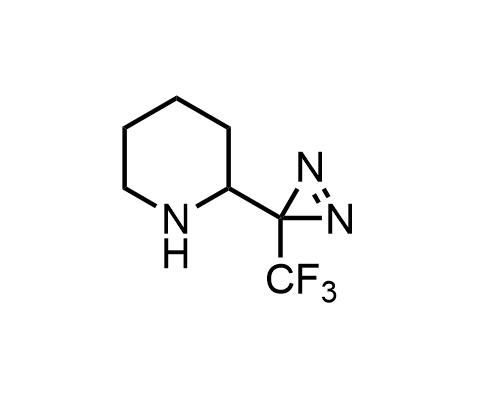
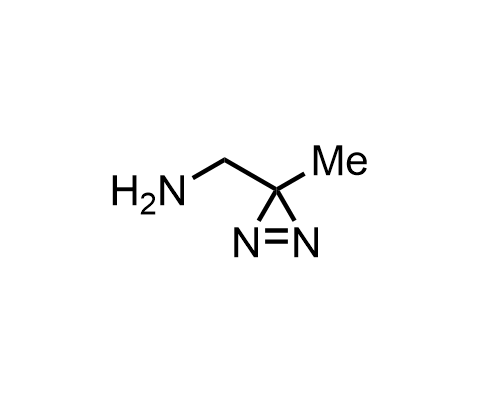
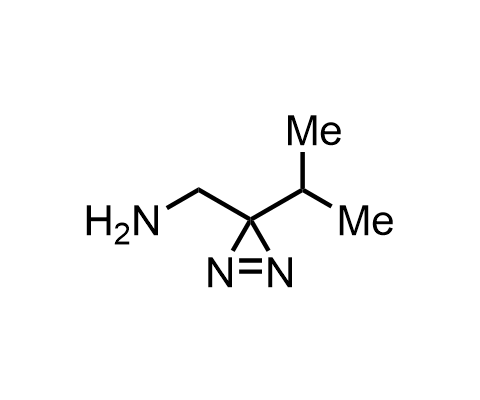
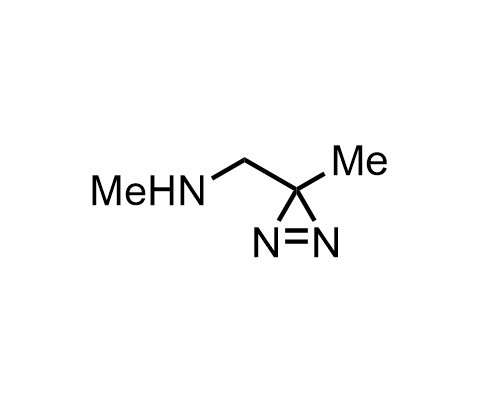
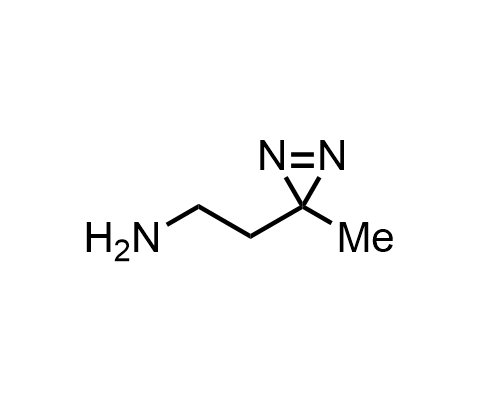
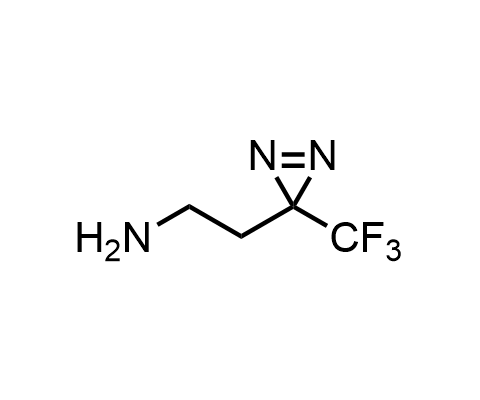
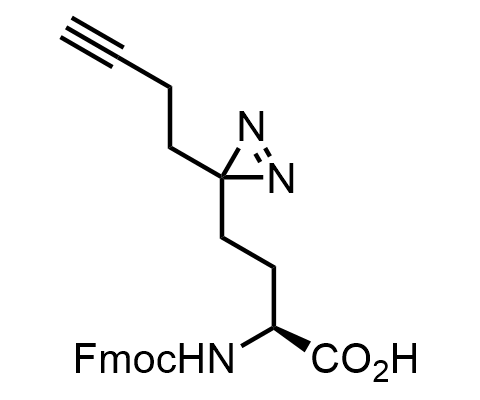
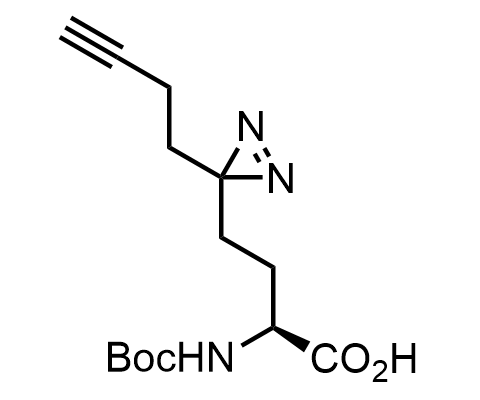
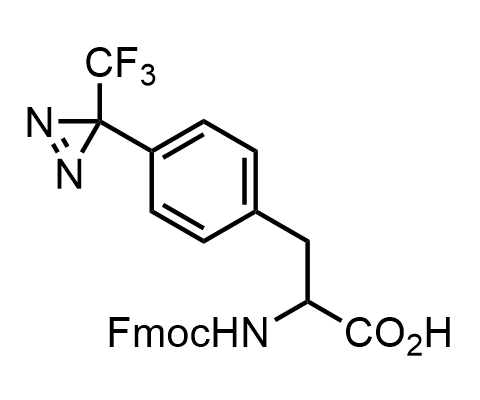
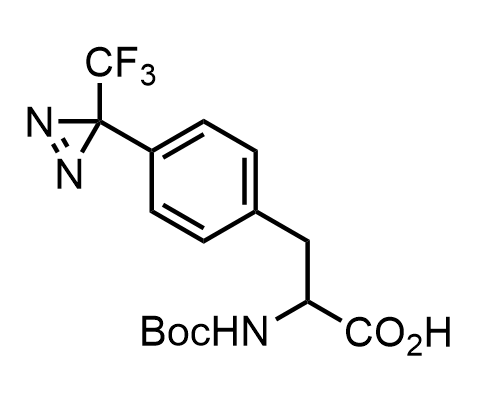
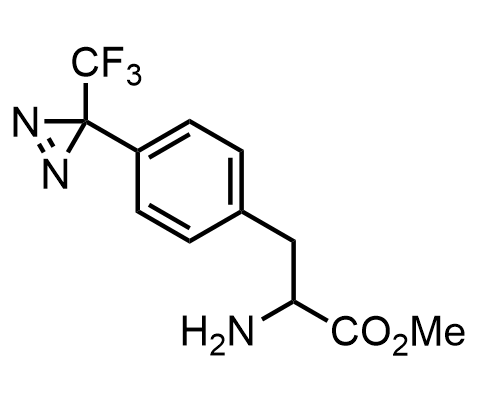
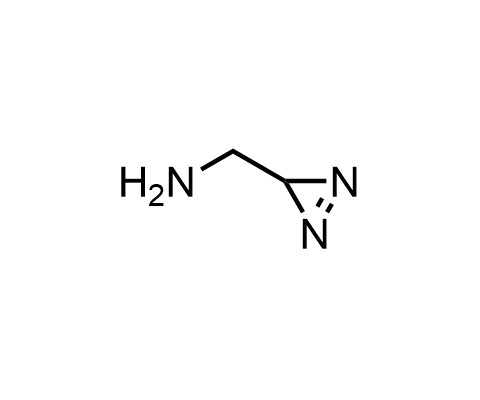
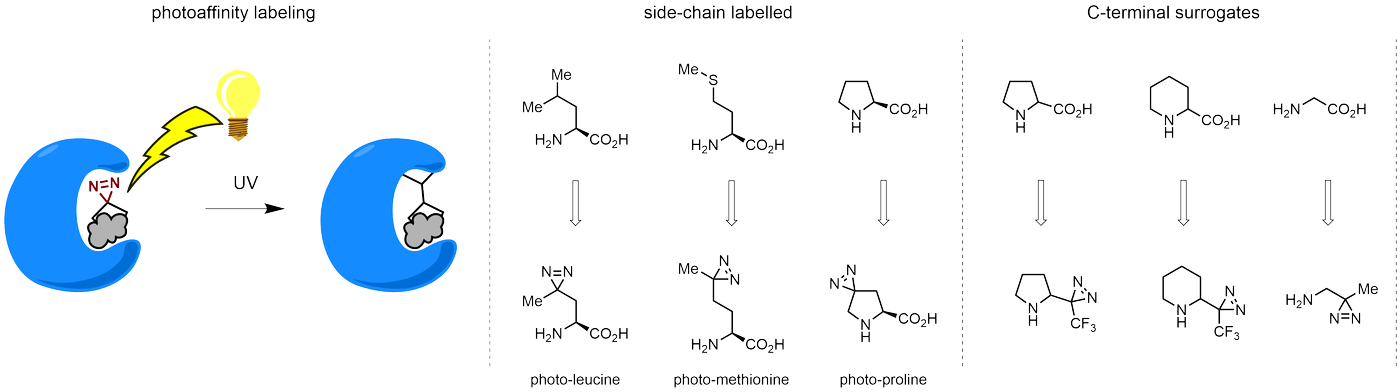
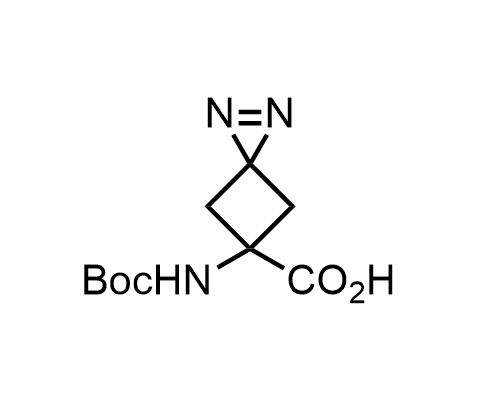Diazirines are chemically stable, small heterocyclic fragments that undergo controlled decomposition upon UV-light irradiation, releasing highly reactive carbene species that quickly bind to spatially proximal molecular fragments. Various diazirines have been used for photoaffinity labeling in the field of proteomics and for mapping protein-ligand interaction sites. Amino acids bearing diazirines in their side chains are usually called photo amino acids, e.g., photo-leucine, photo-methionine, and photo-proline. Enamine offers photo-amino acids with diazirines in the side chains, as well as amino acid surrogates with diazirine groups replacing the backbone groups and diazirine tags.
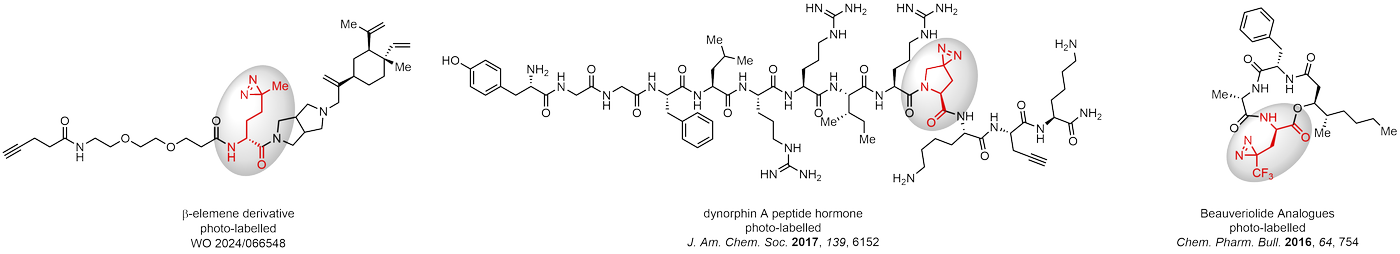
Concept
Download SD file
Download PDF file
We offer
More than 20 photo-amino acids and amino acid surrogates from stock on a 5-10 g scale.