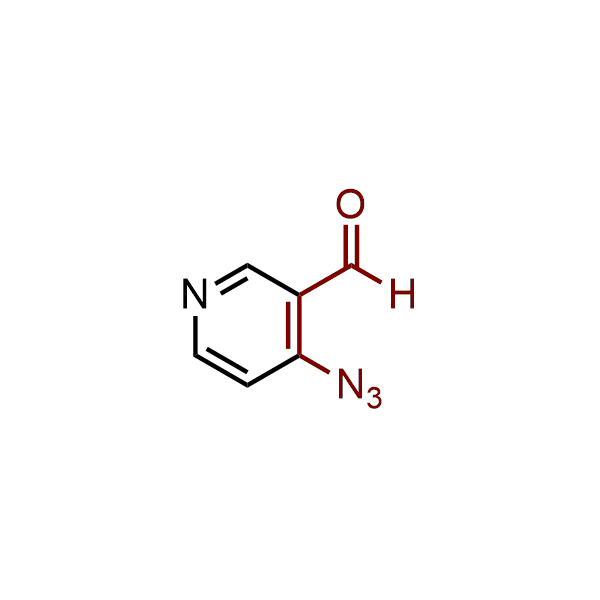
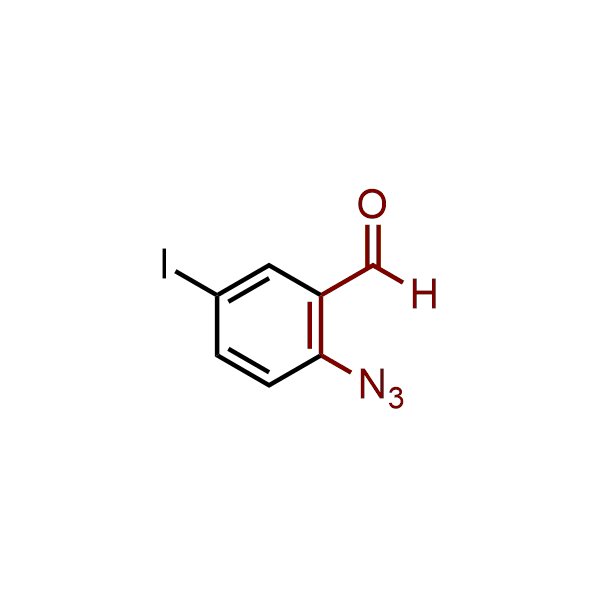
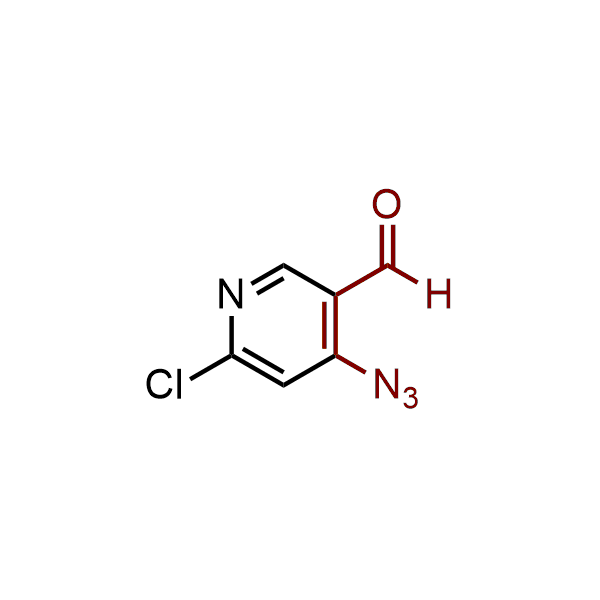
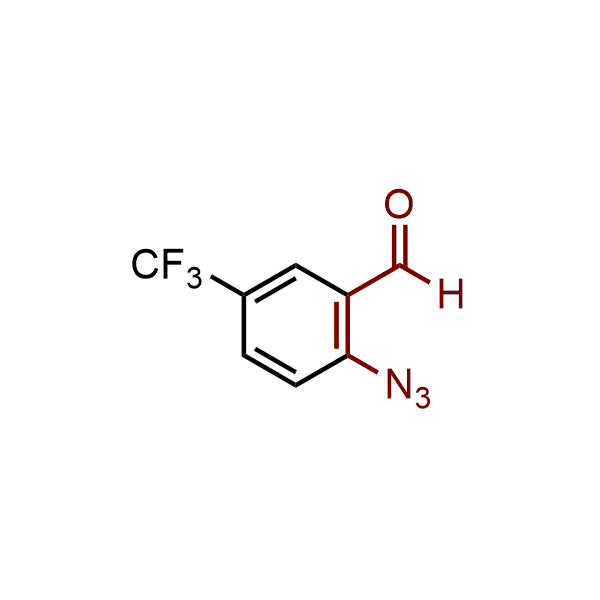
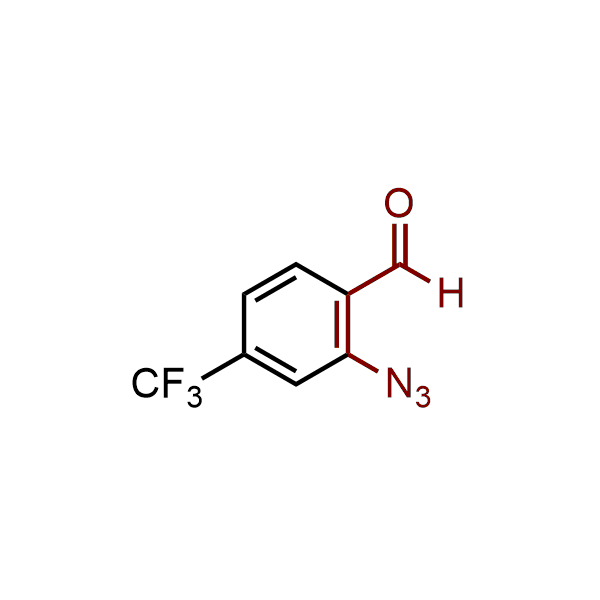
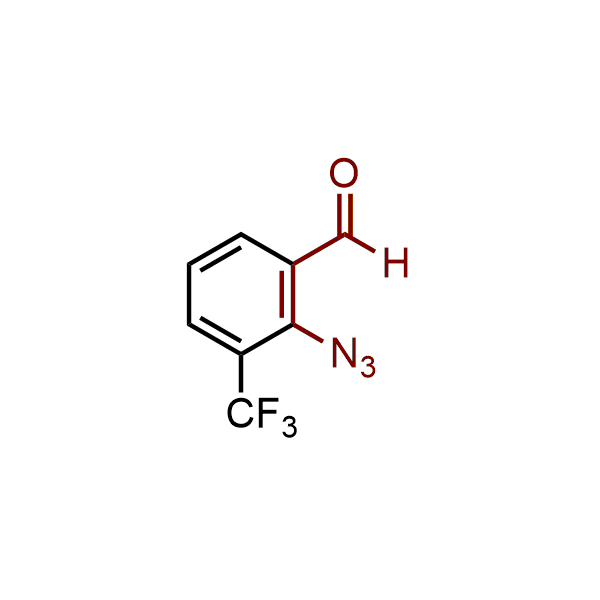
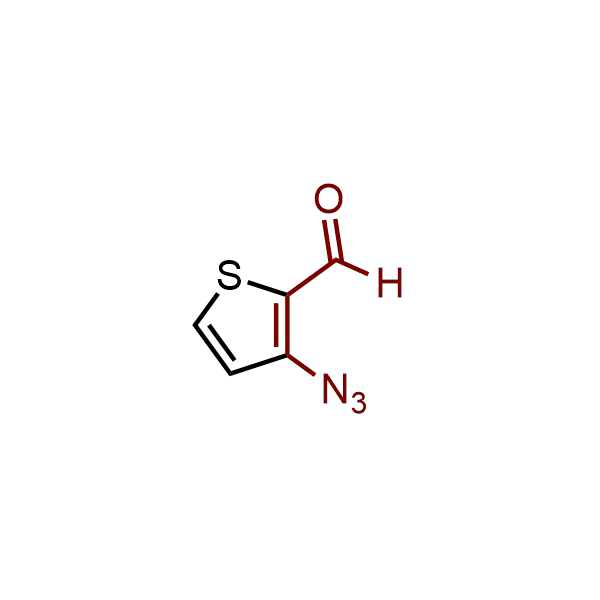
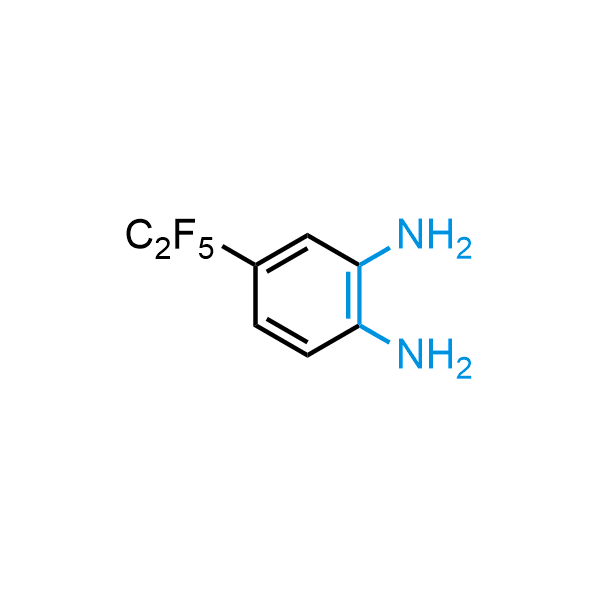
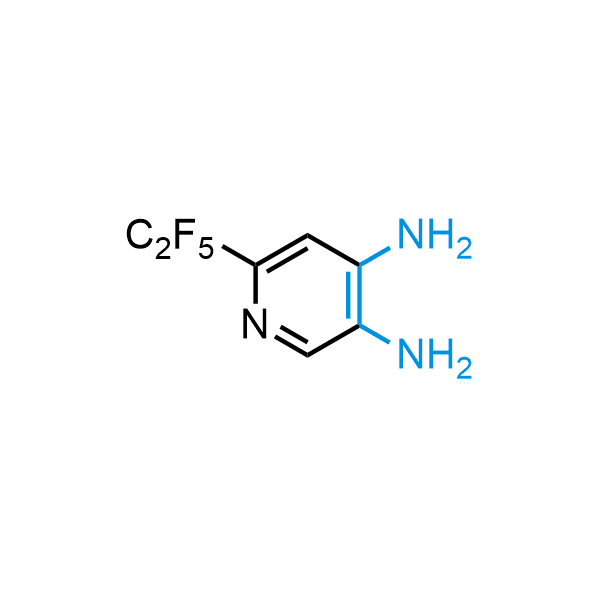
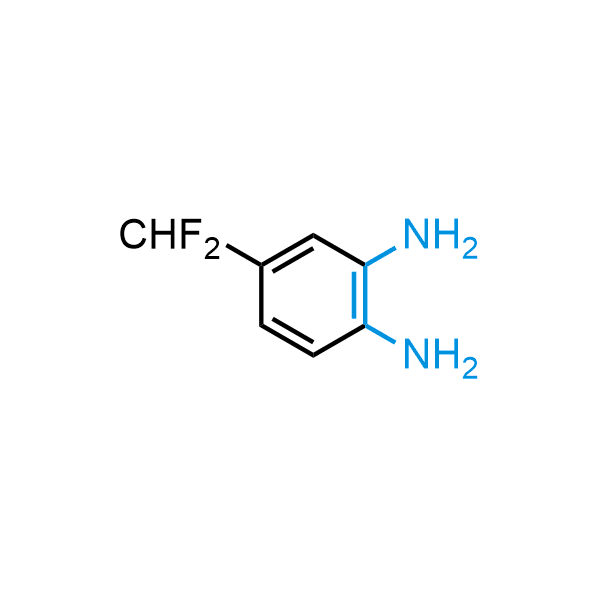
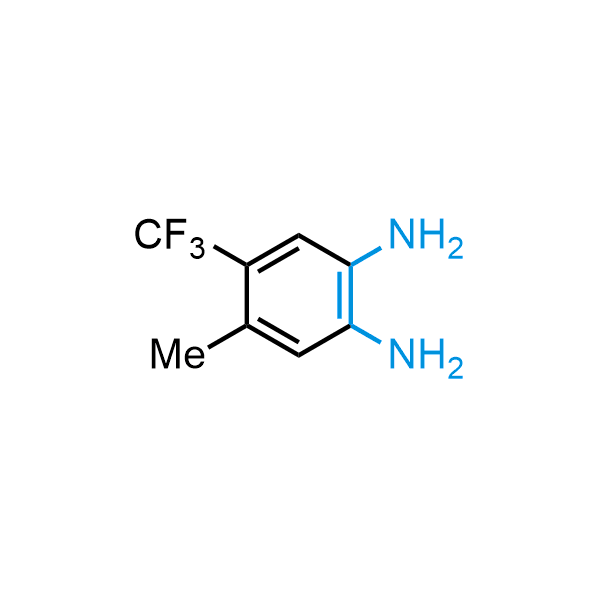
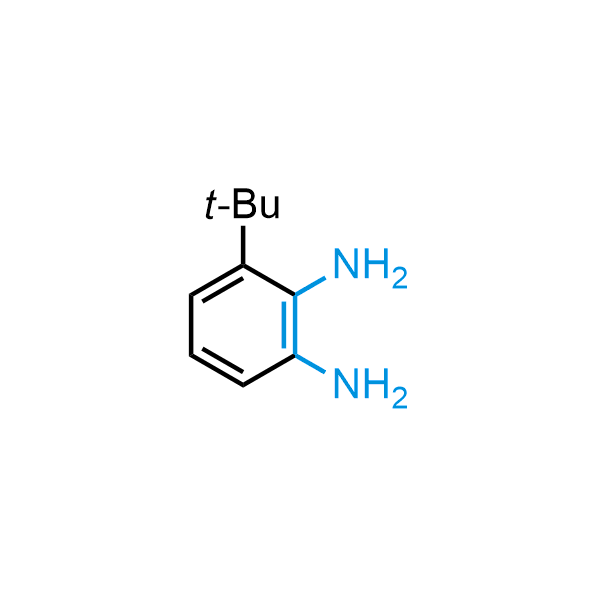
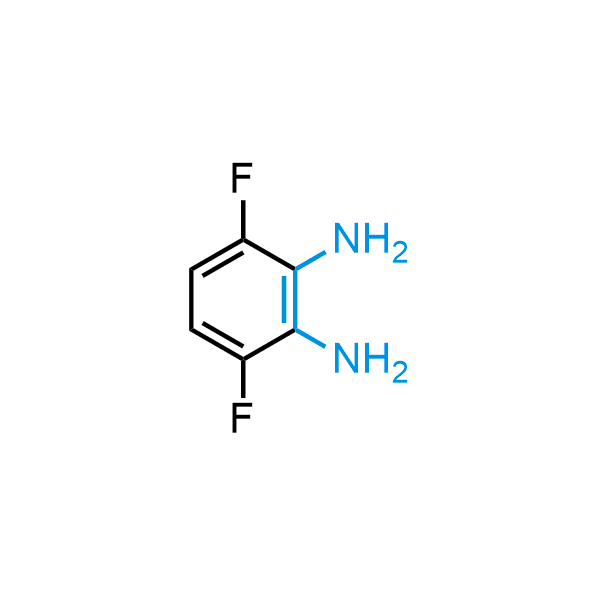
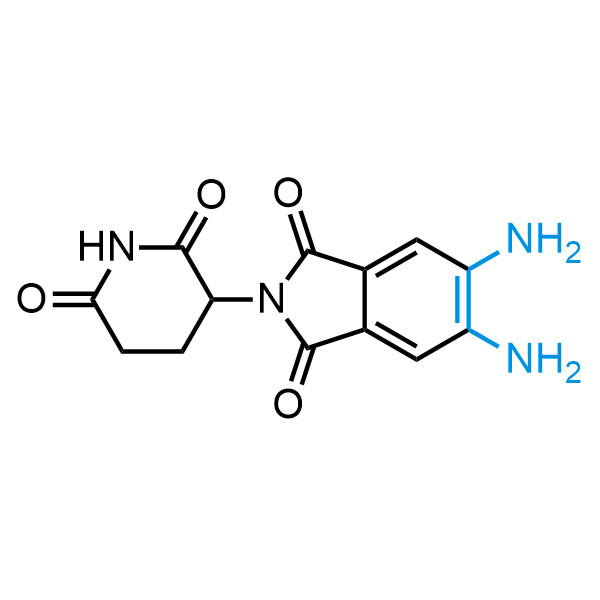
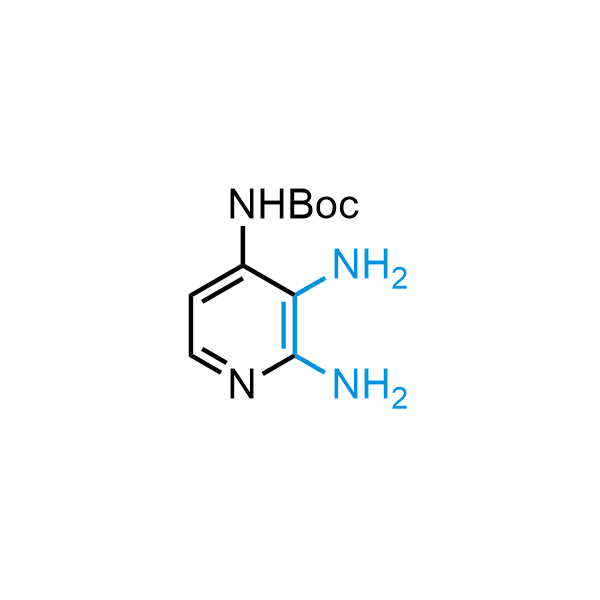
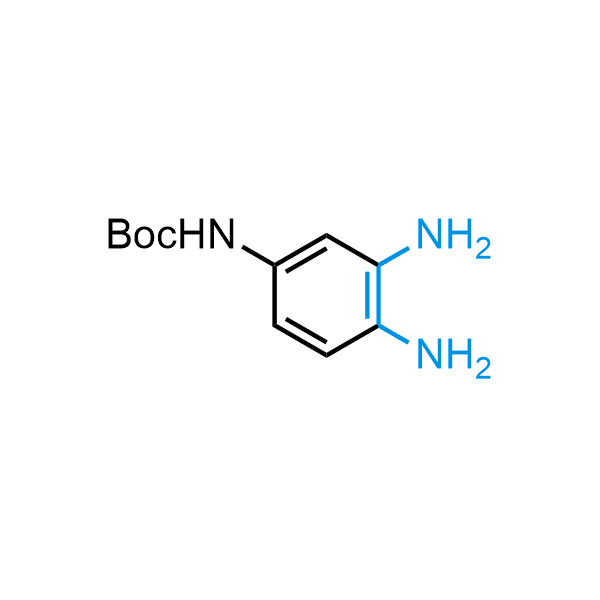
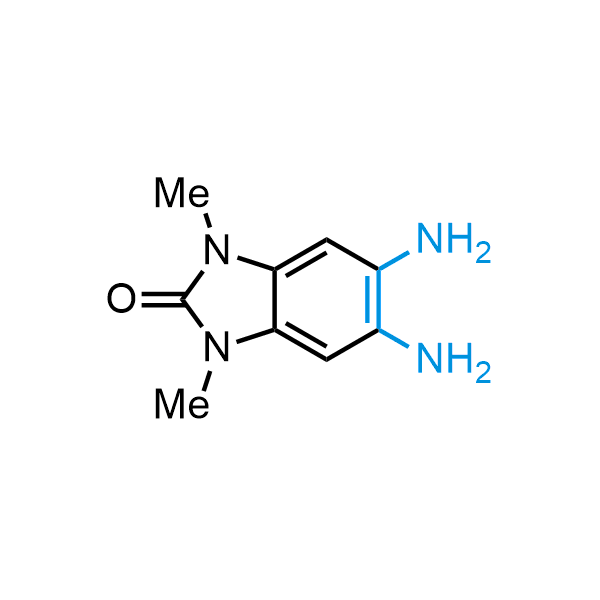
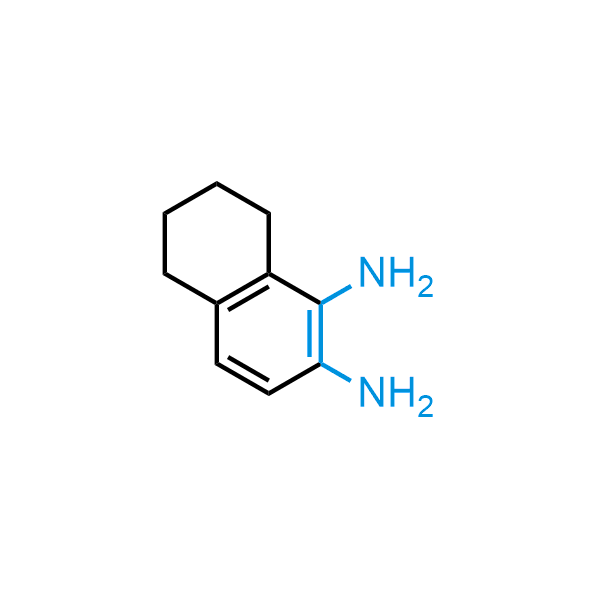
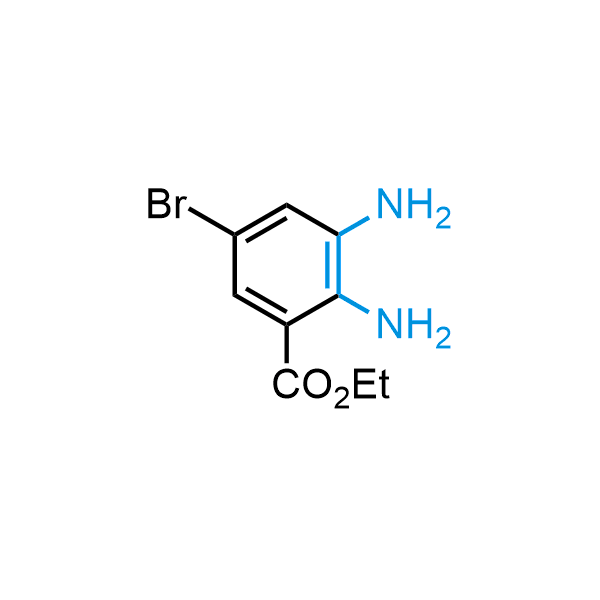
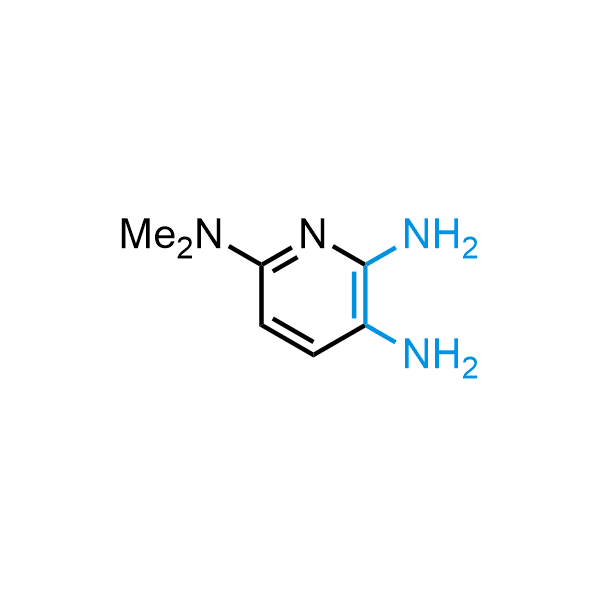
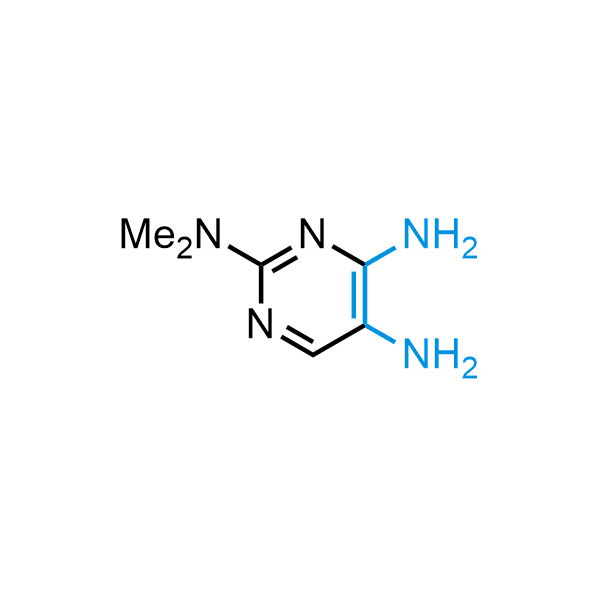
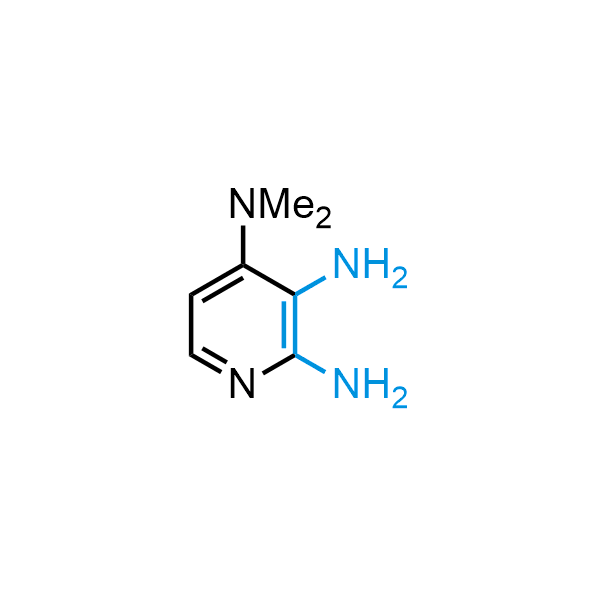
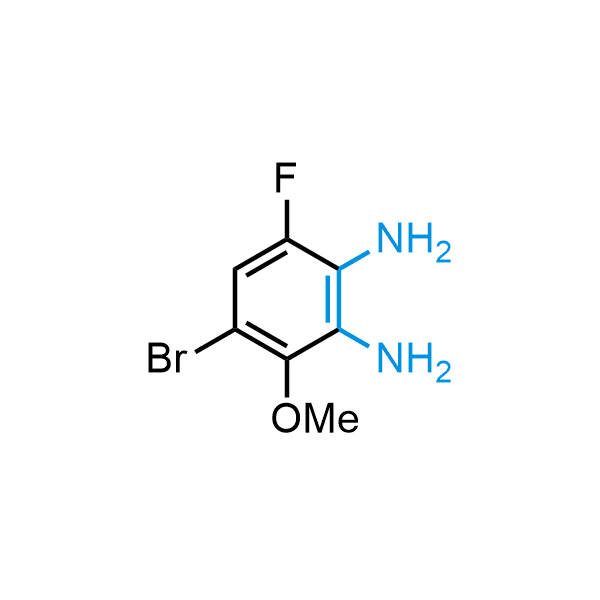
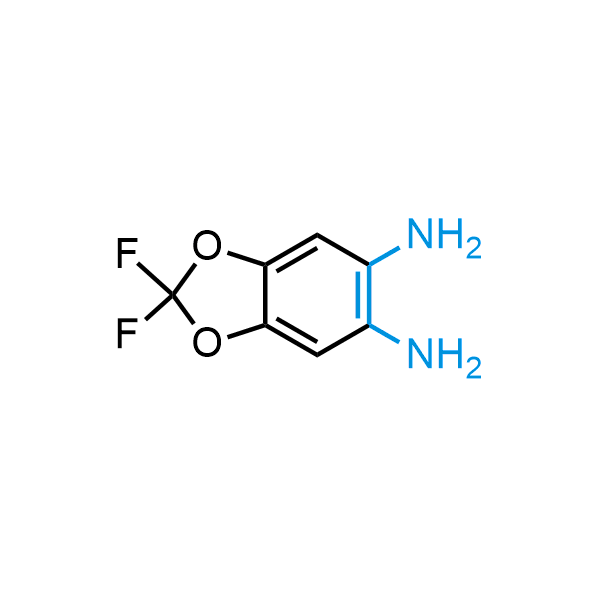
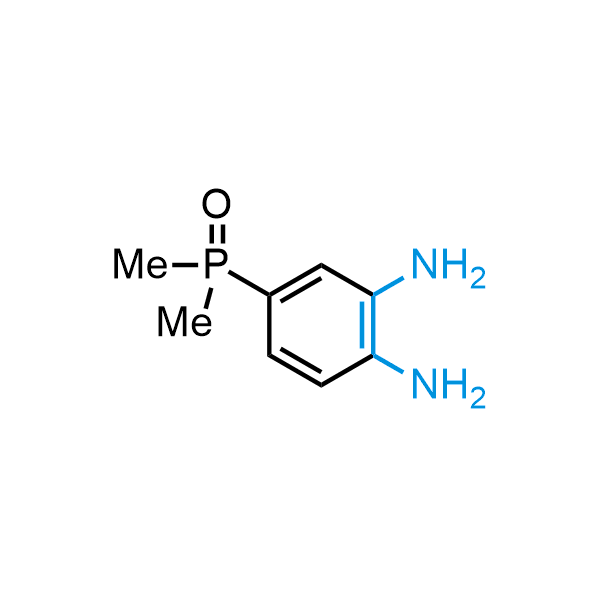
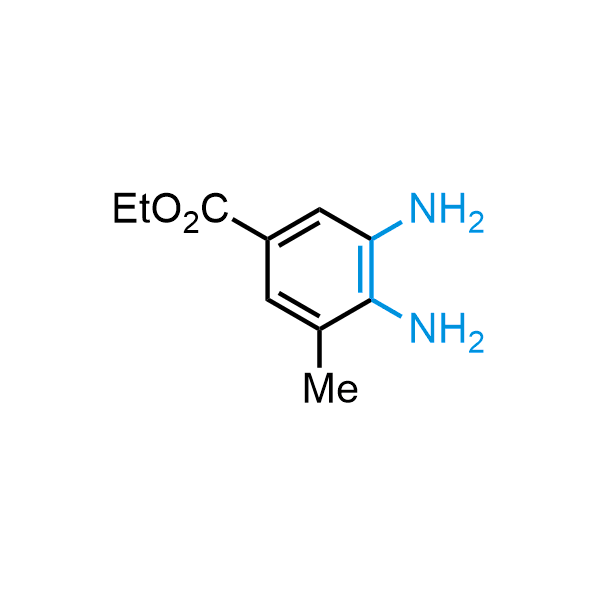
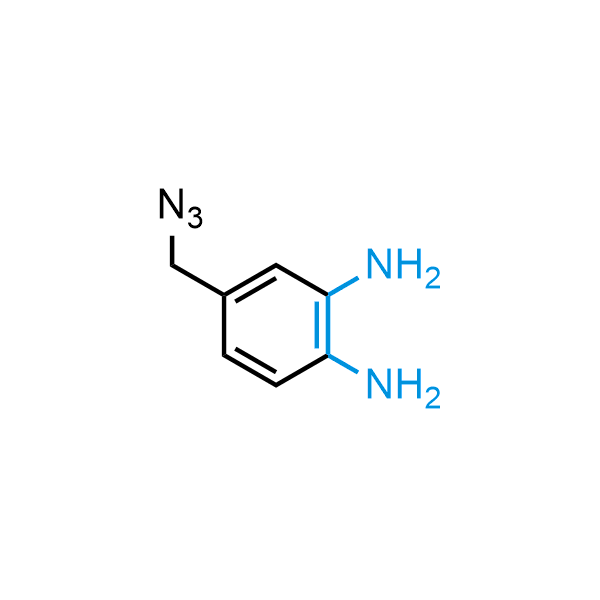
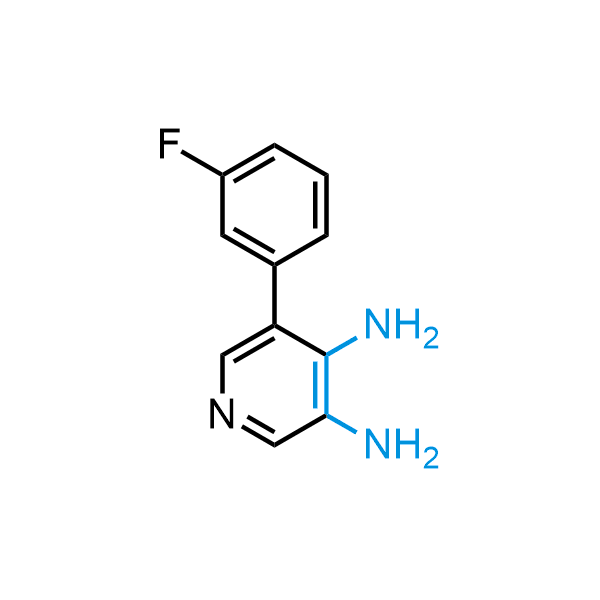
The construction of complex screening libraries requires a series of synthetic chemical reactions, culminating in a versatile, robust, and selective reaction for the high-throughput assembly of the final molecules. Recently, researchers at Merck demonstrated that the heterocyclic assembly of benzimidazoles achieves parallel chemistry libraries with success rates (77%) comparable to the most common late-stage reactions, such as Suzuki and Buchwald-Hartwig cross-coupling. Their approach involves the late-stage transformation of ortho-diamino aromatics and ortho-azidoaldehydes, yielding azabenzimidazoles and azaindazoles, respectively. The resulting heterocyclic cores are among the most prevalent in drug structures.
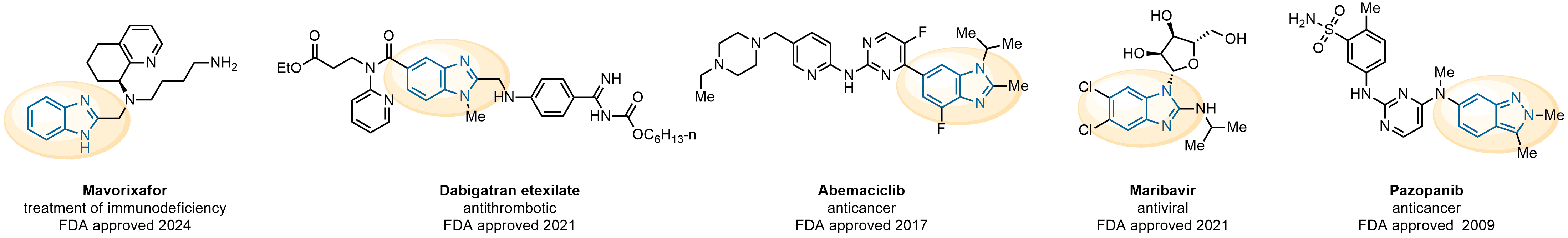
Reaction
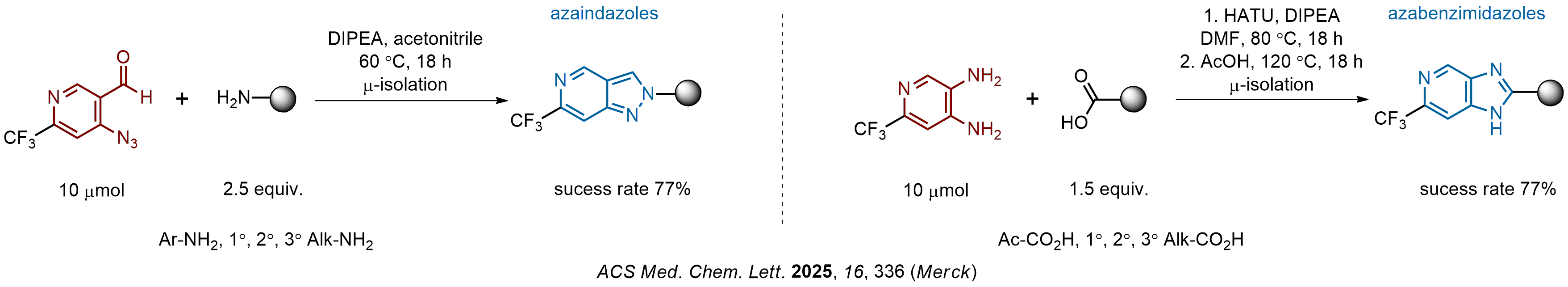
We offer
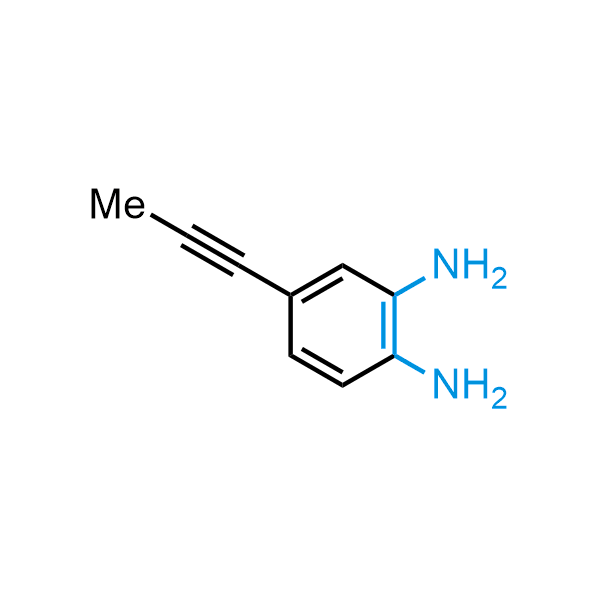
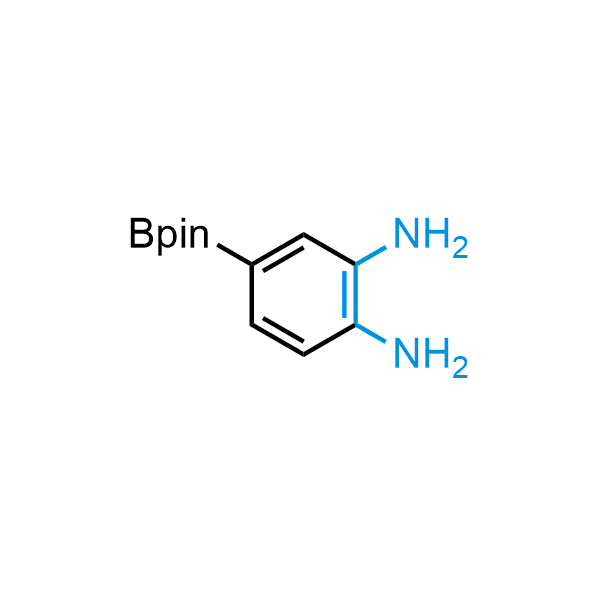
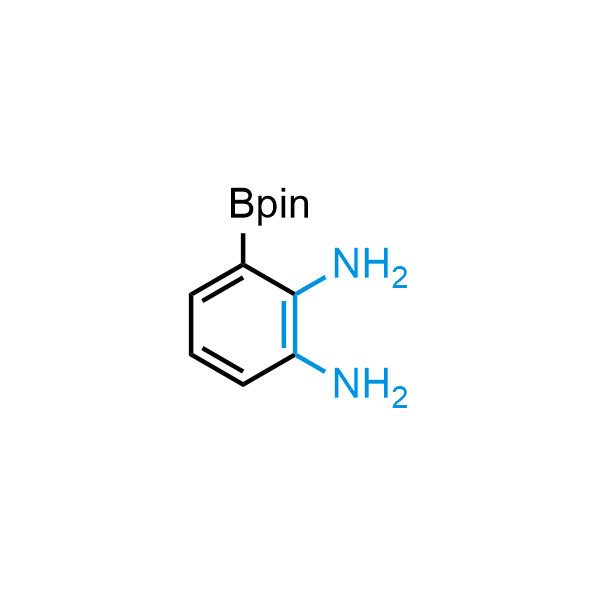
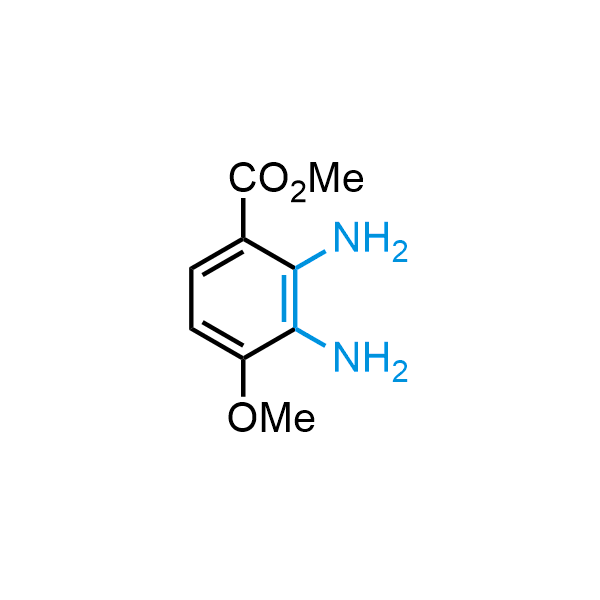
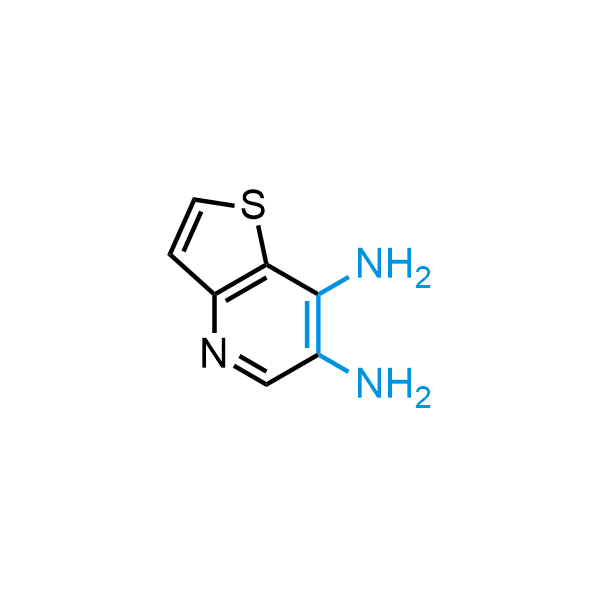
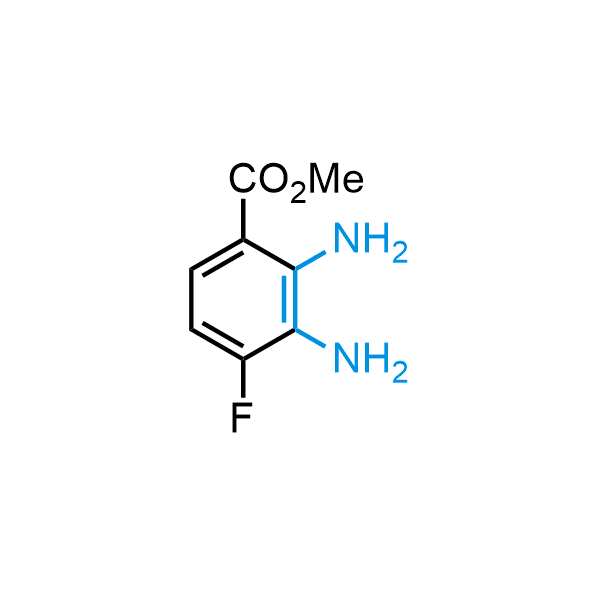
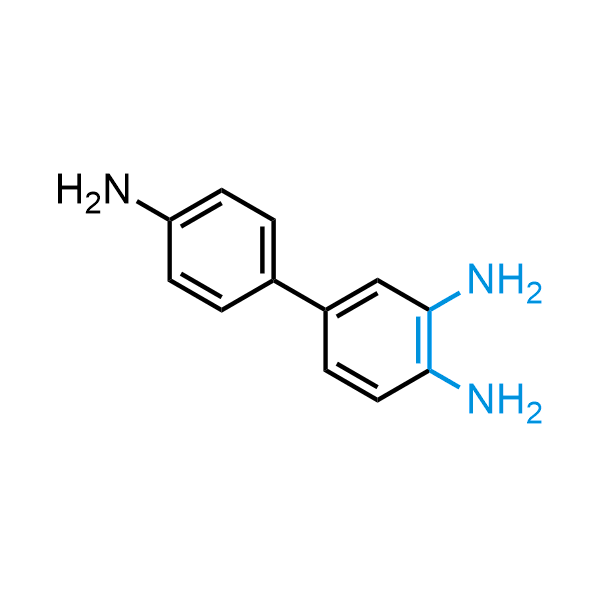
12 ortho-azidoaldehydes and over 250 ortho-arylenediamines from stock on 5-10 gram scale.