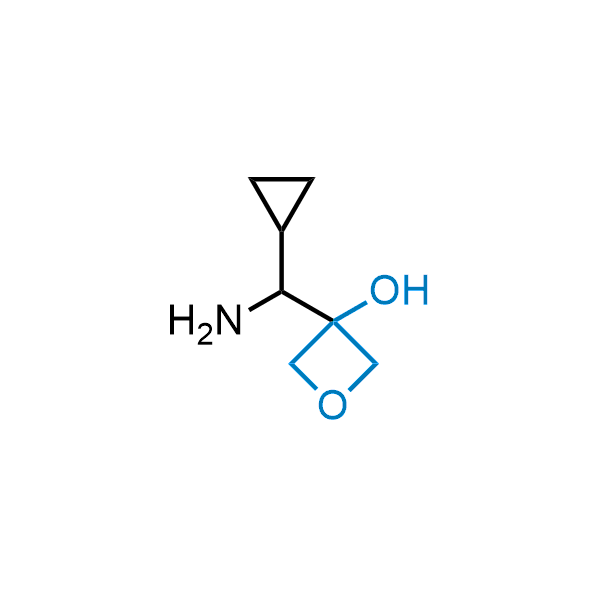
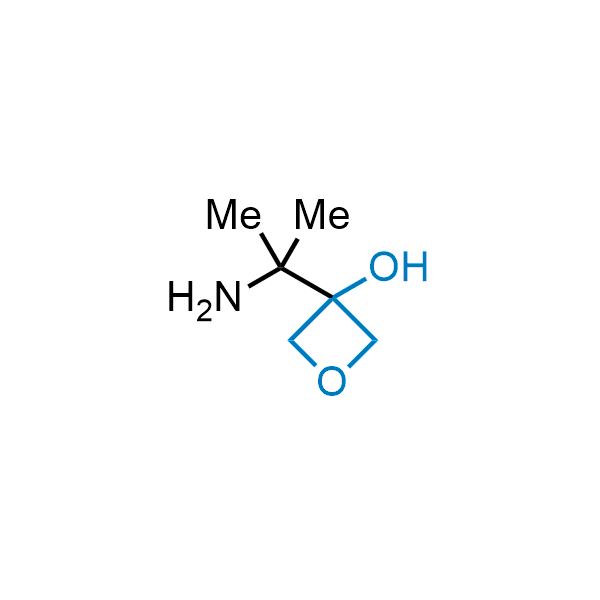
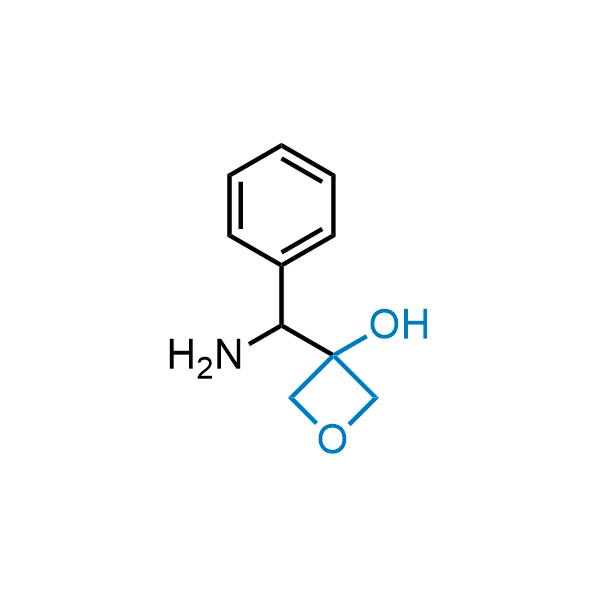
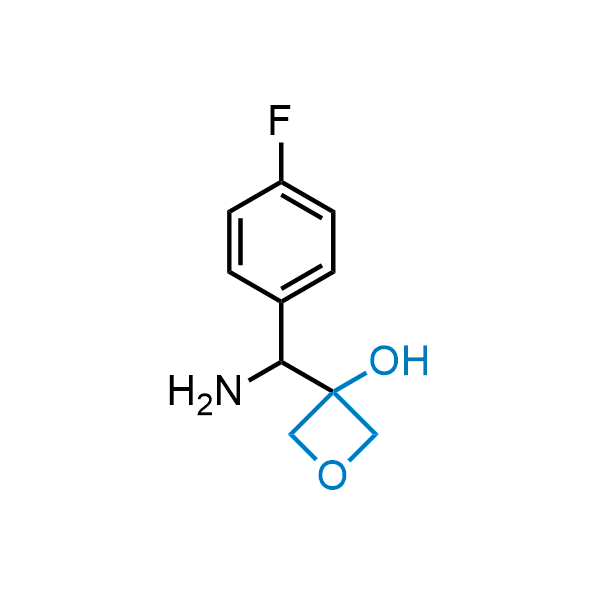
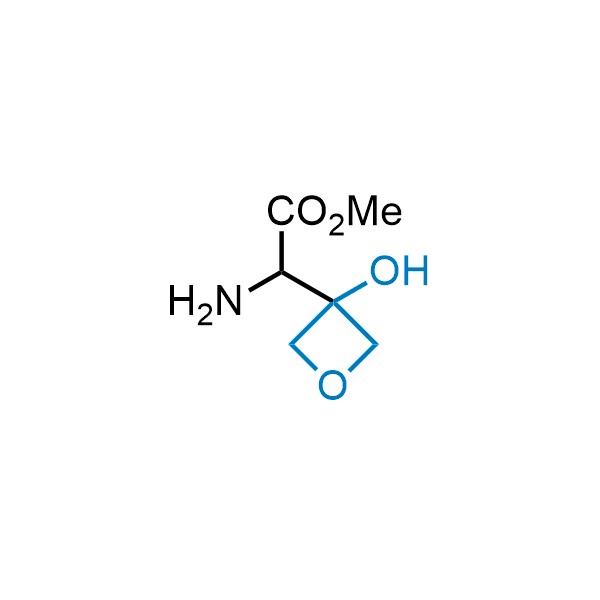
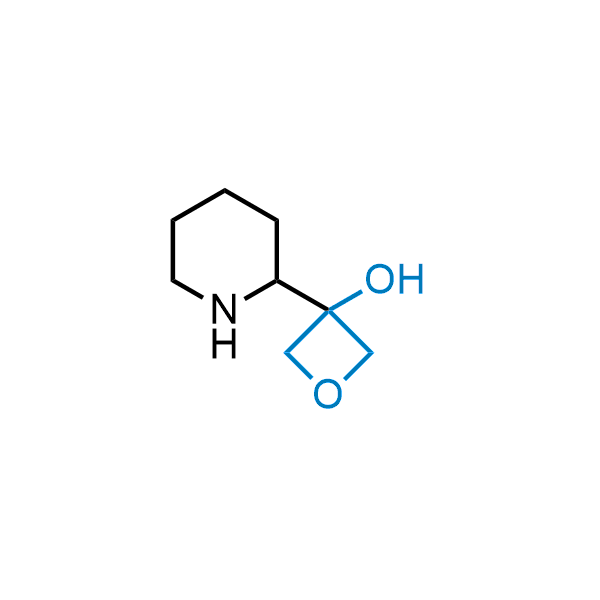
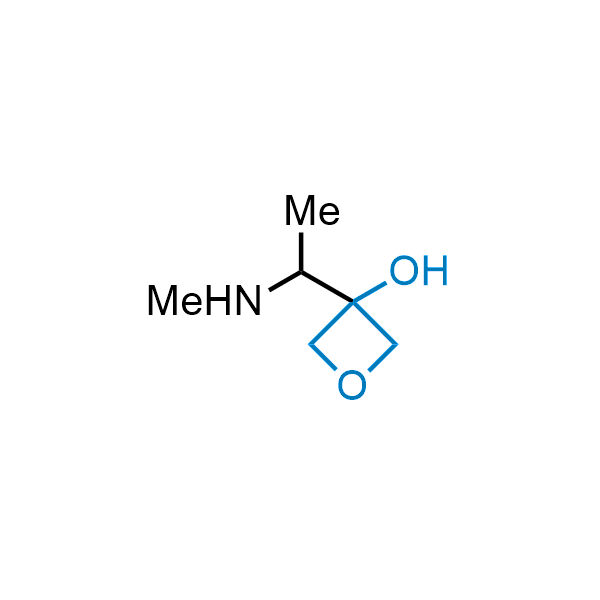
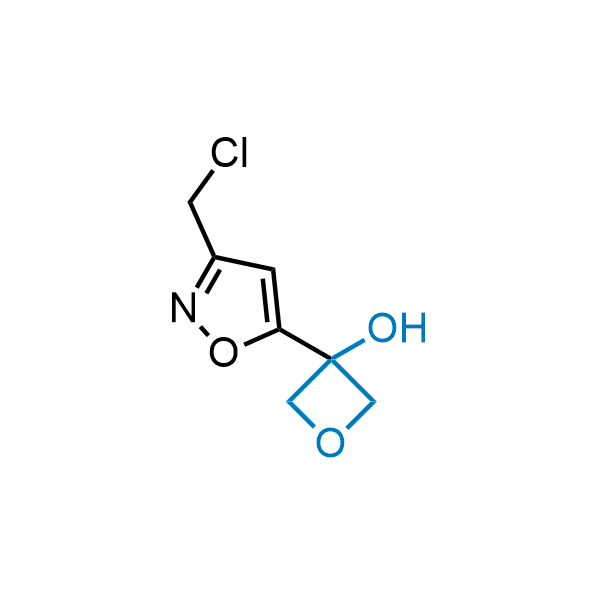
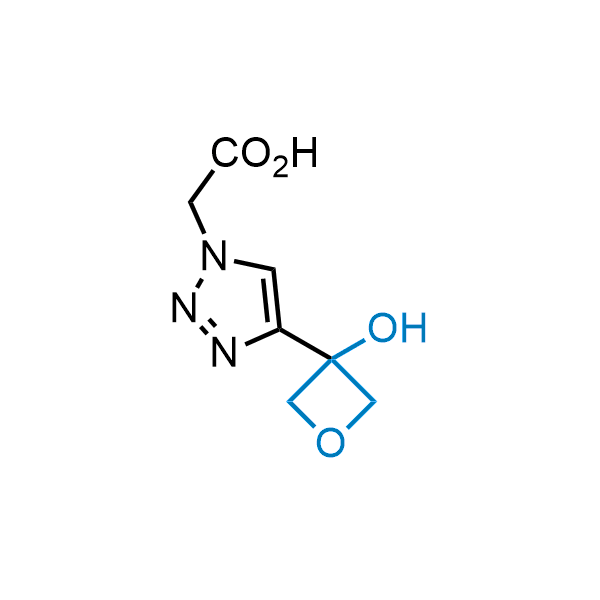
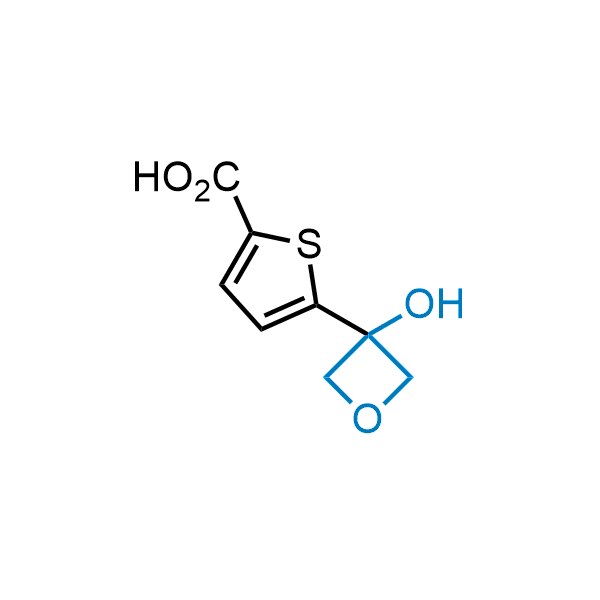
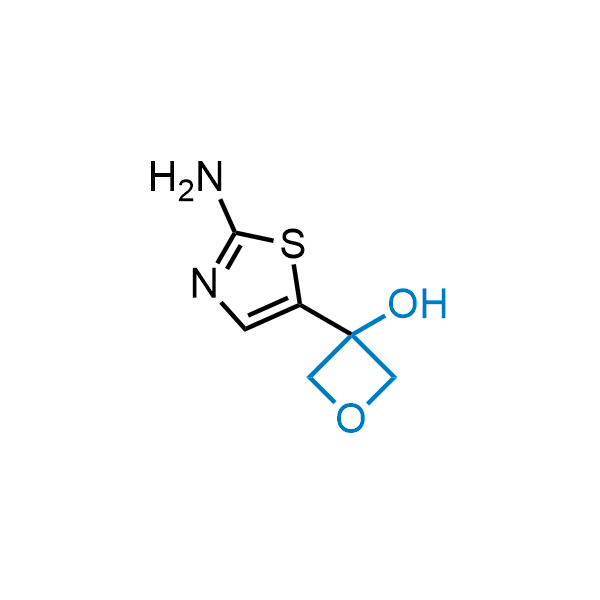
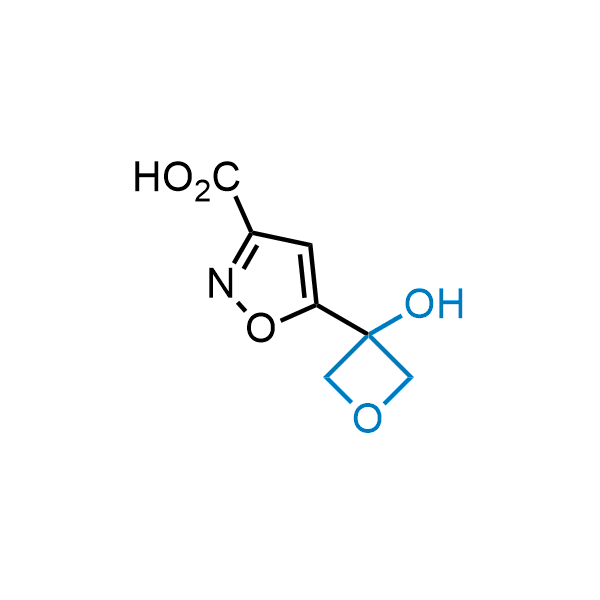
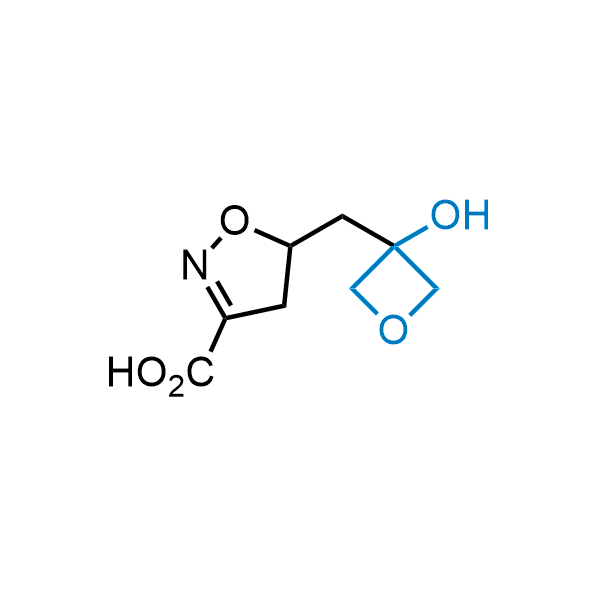
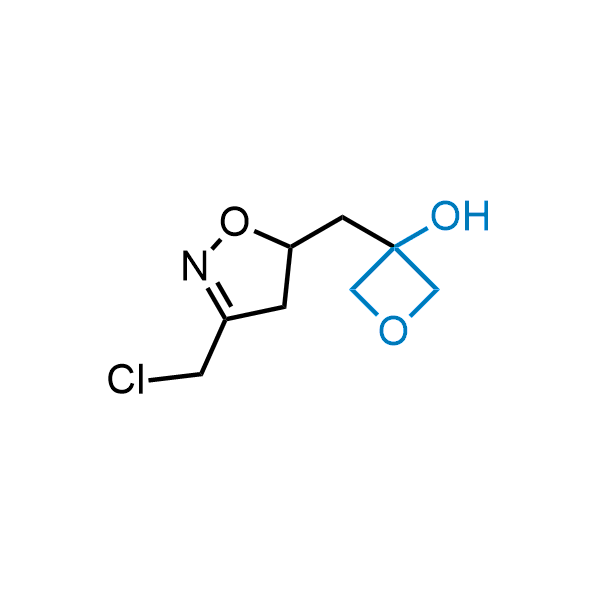
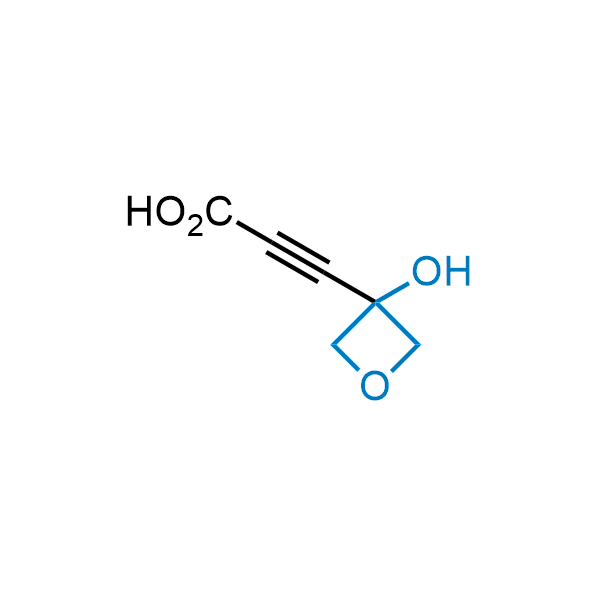
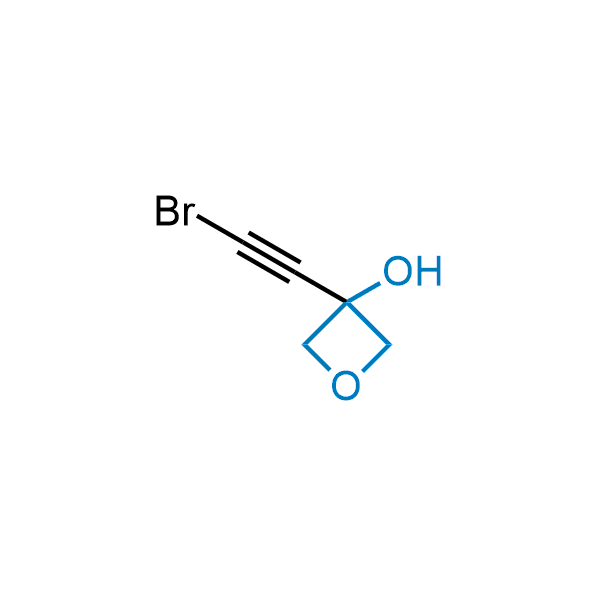
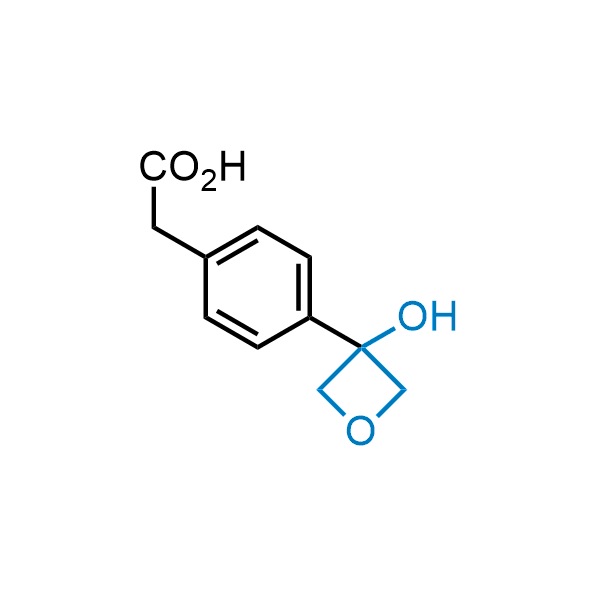
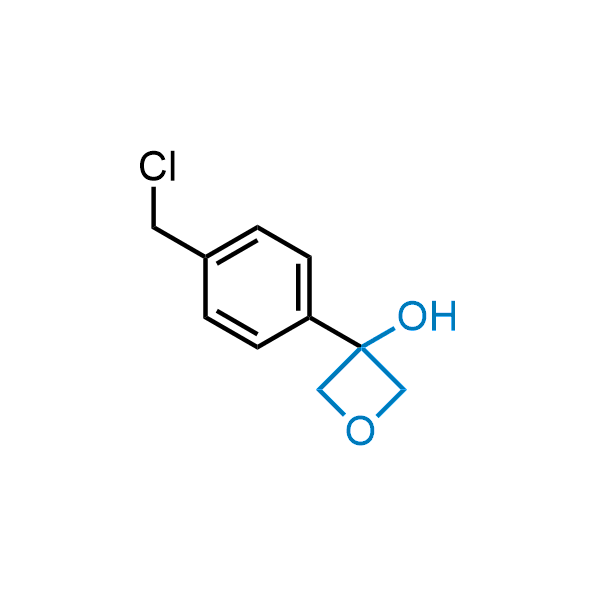
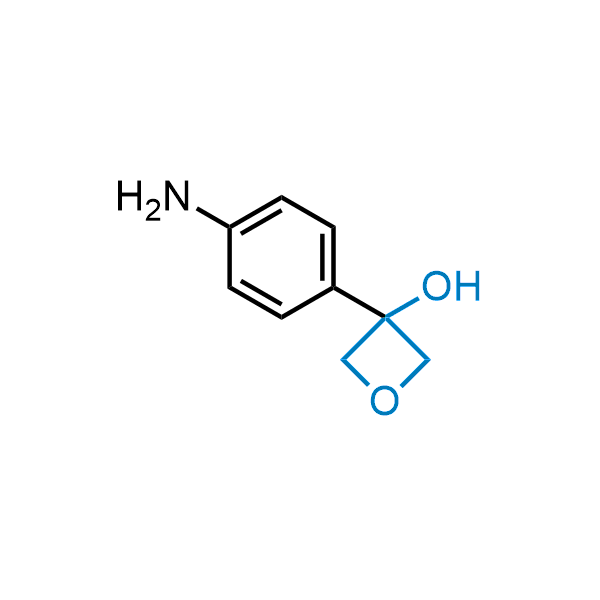
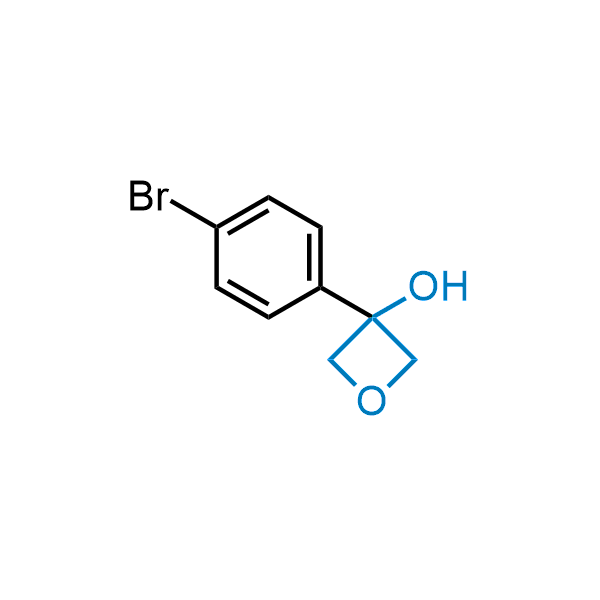
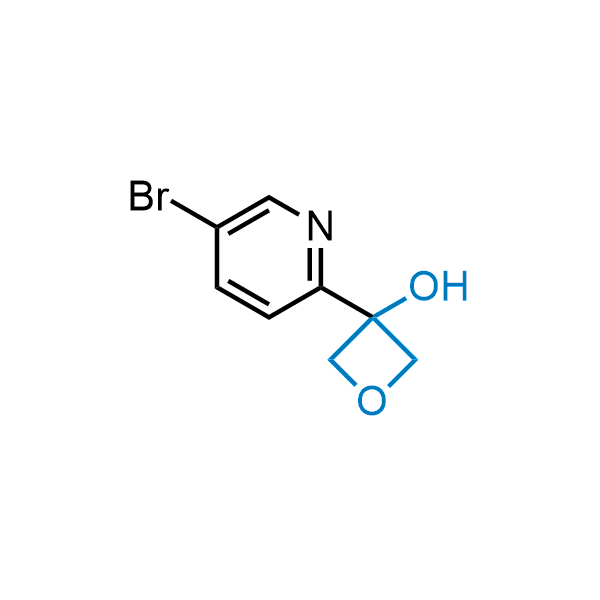
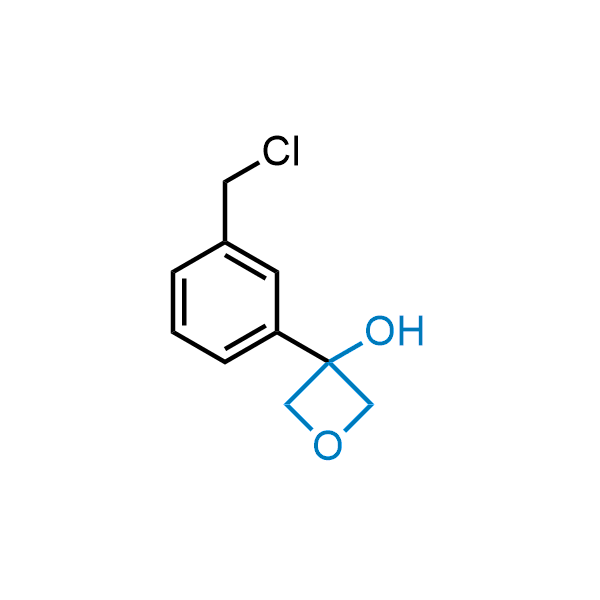
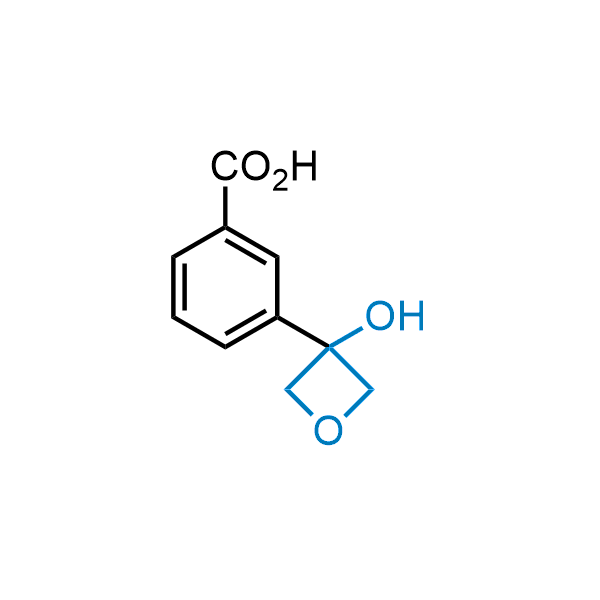
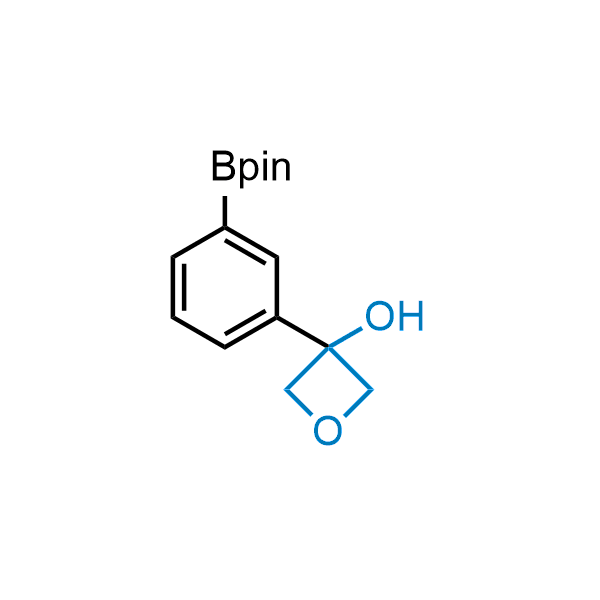
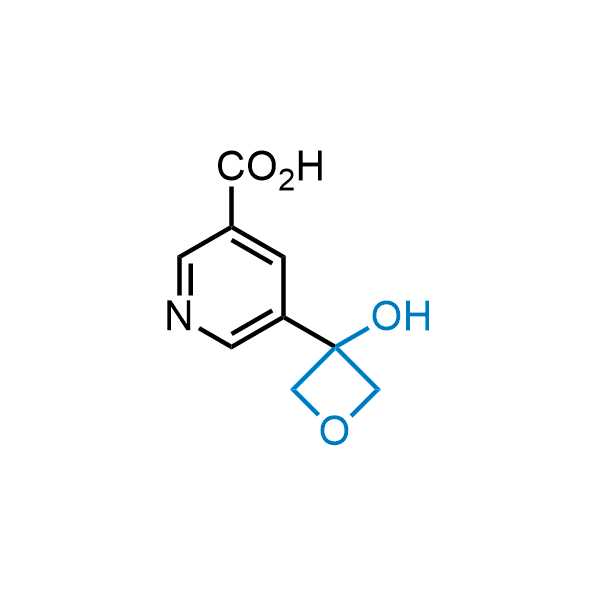
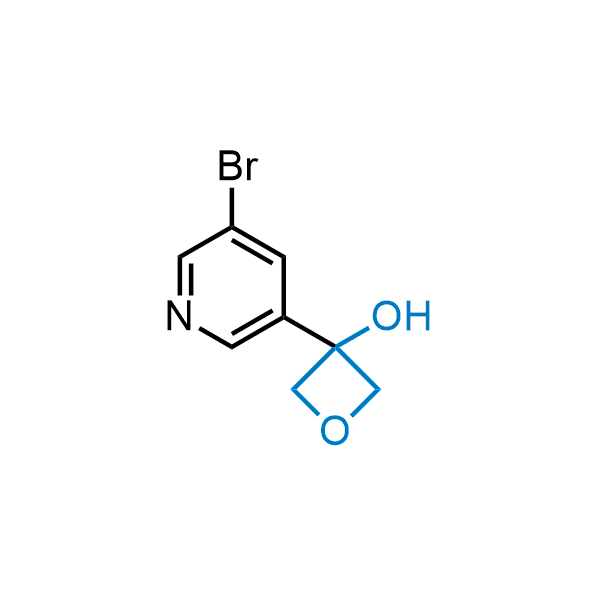
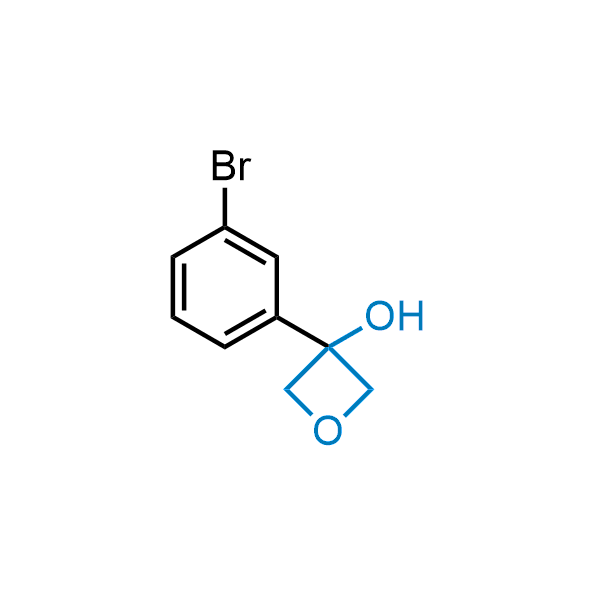
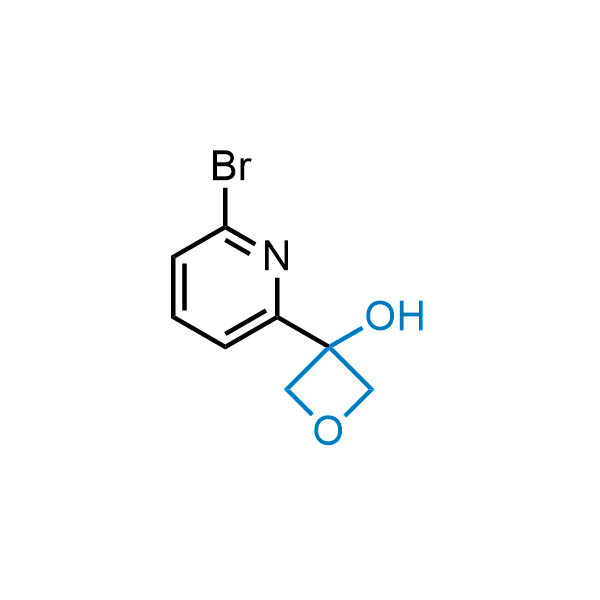
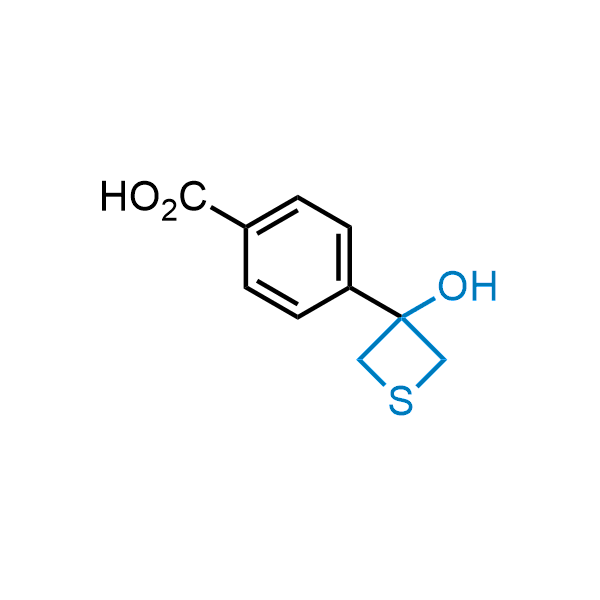
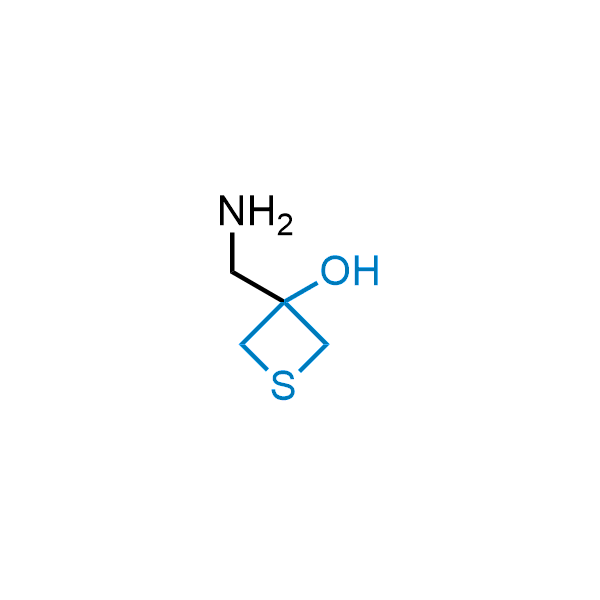
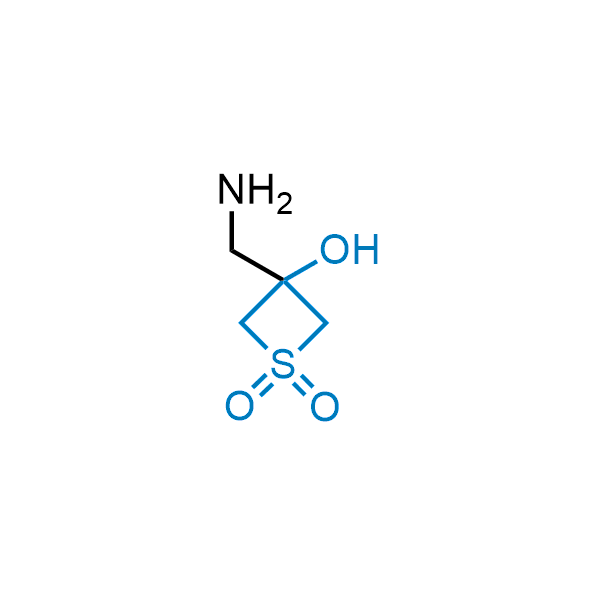
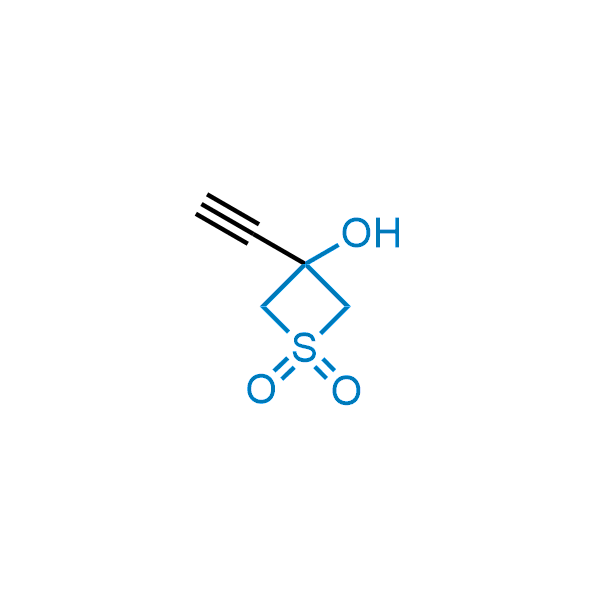
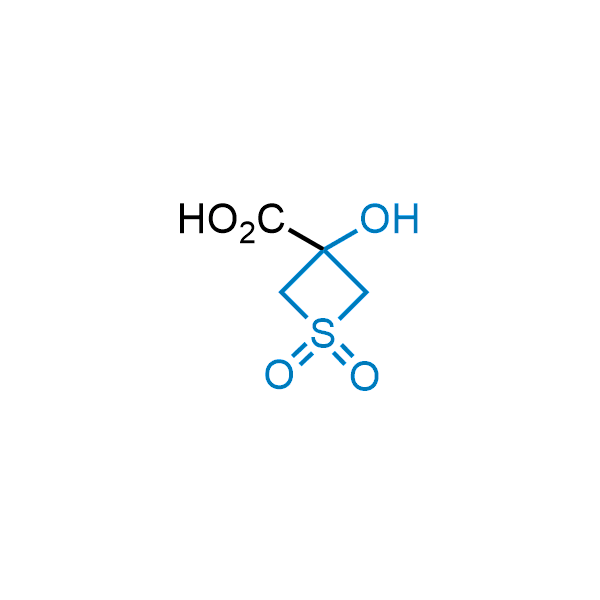
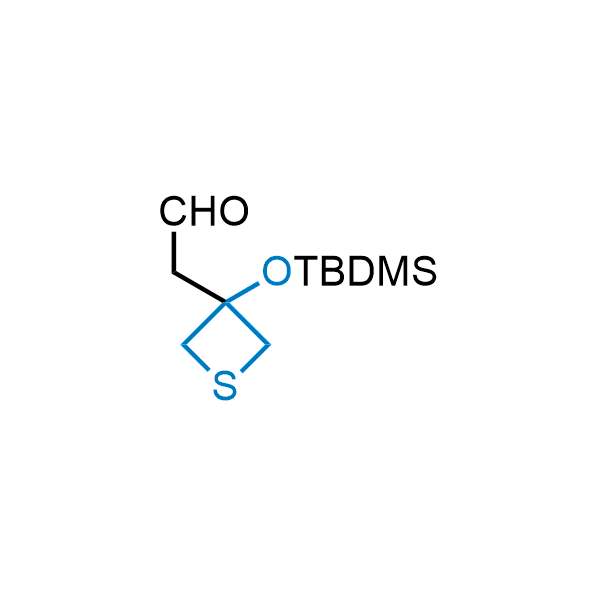
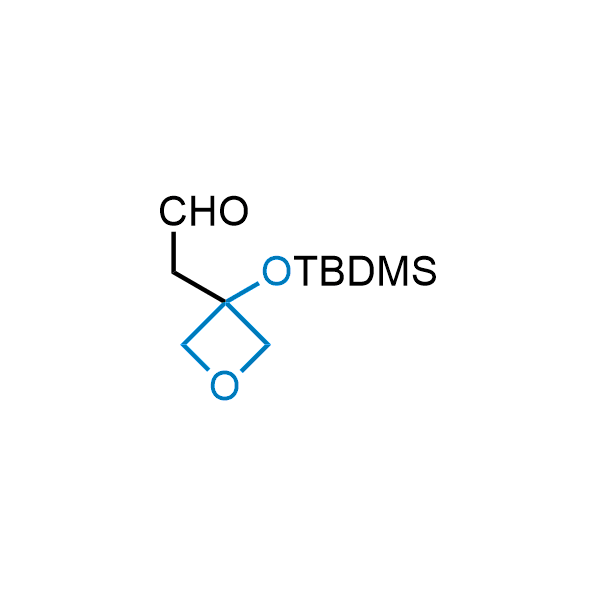
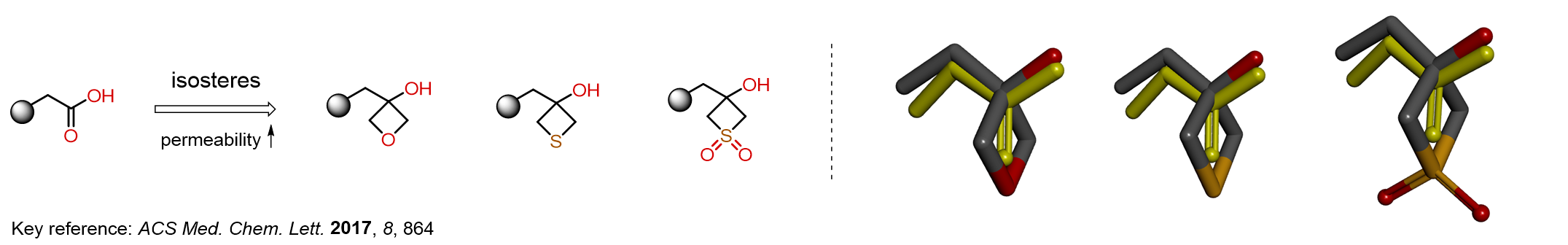
Replacing carboxylic acids with bioisosteres can enhance permeability and minimize unwanted metabolic transformations, such as the formation of acyl glucuronides and coenzyme A esters. Beyond classical analogues like tetrazoles, aliphatic alternatives have also been developed. Showcasing this concept, oxetan-3-ol and thietan-3-ol fragments have been evaluated as carboxylic acid isosteres, demonstrating both improved permeability and retention of target potency.
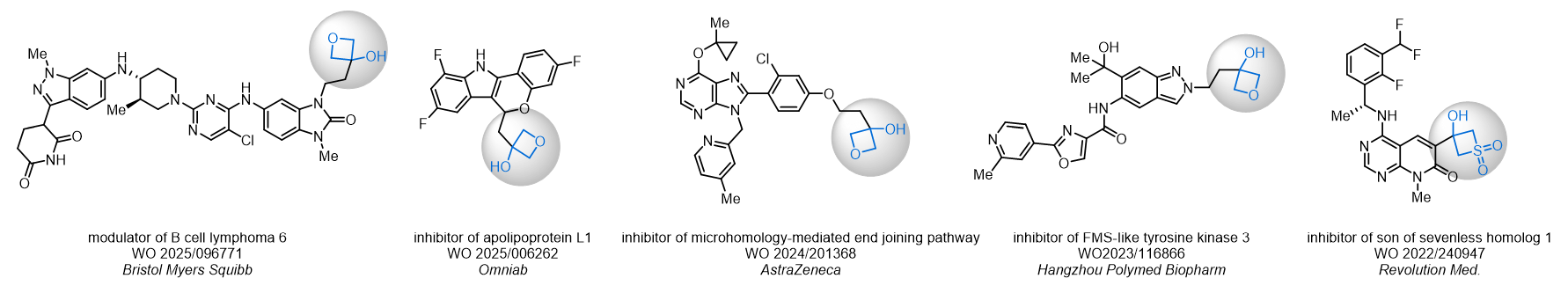
Concept
Download SD files
Download PDF file
We offer
Over 100 oxetanol/thietanol carboxylic isosteres from stock on 5-10 gram scale.