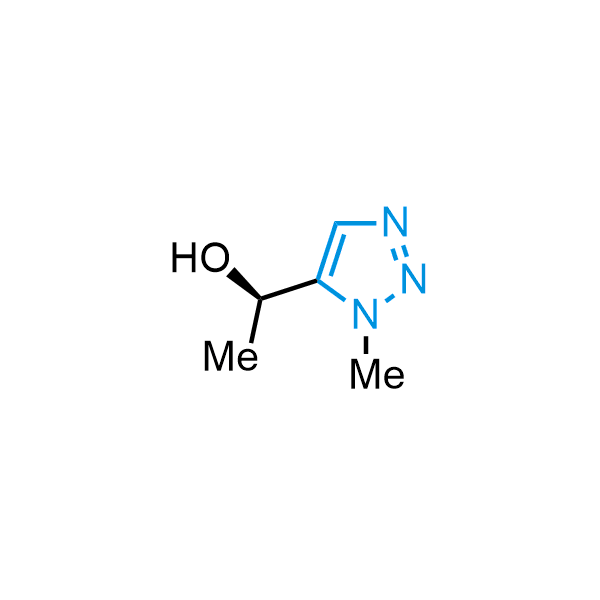
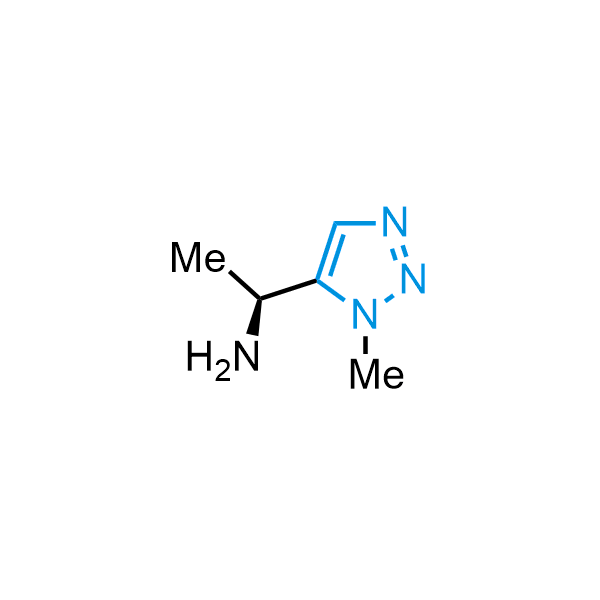
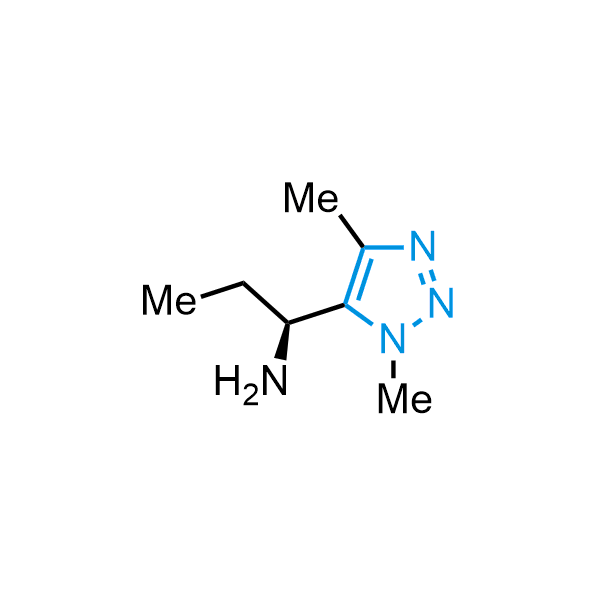
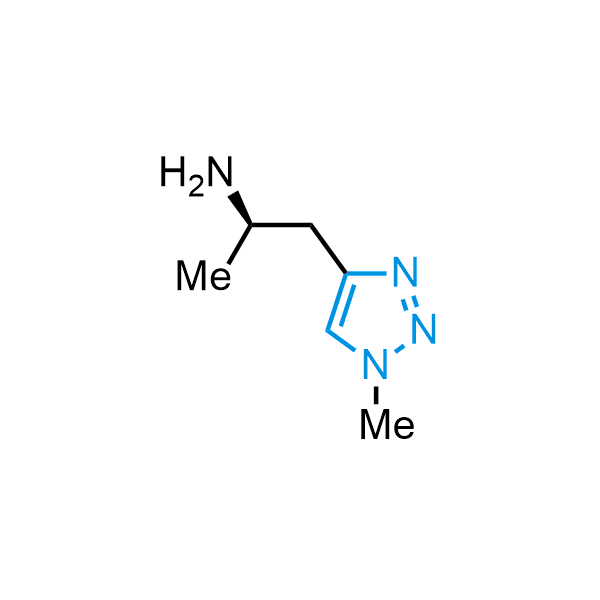
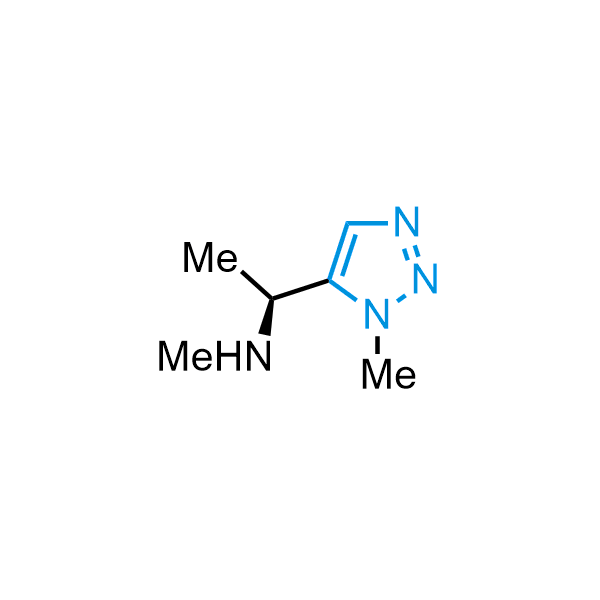
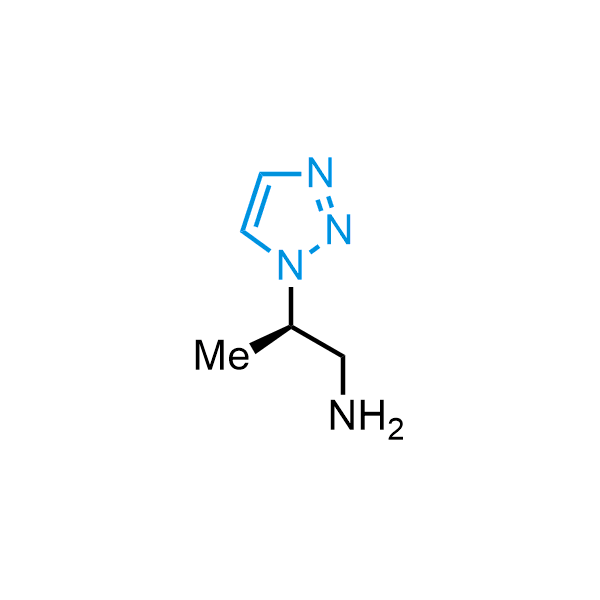
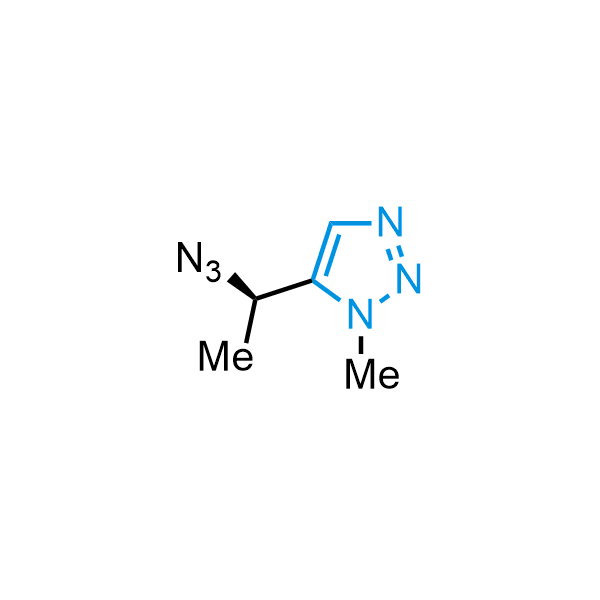
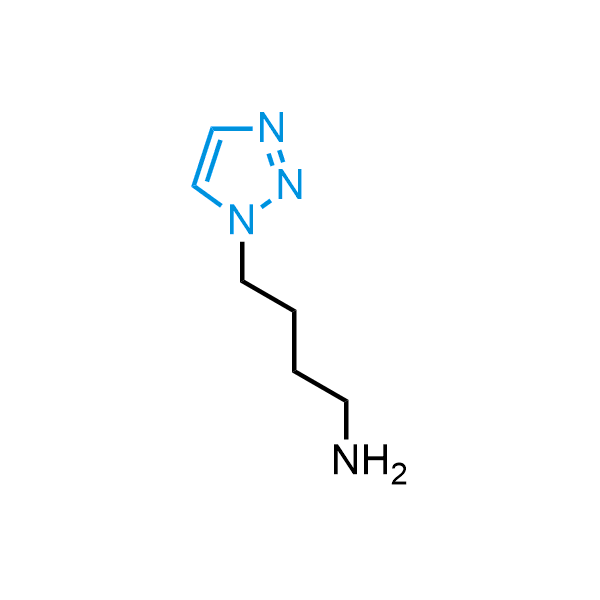
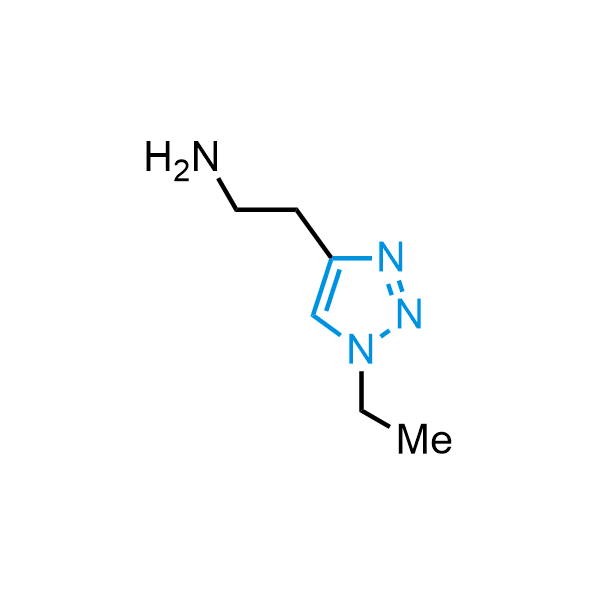
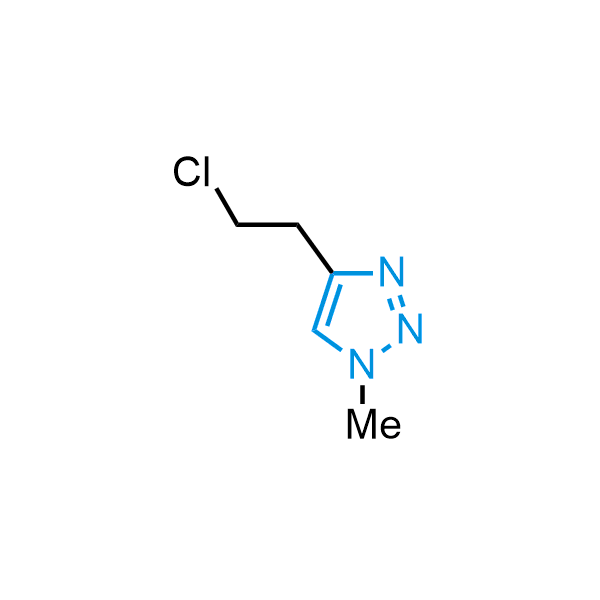
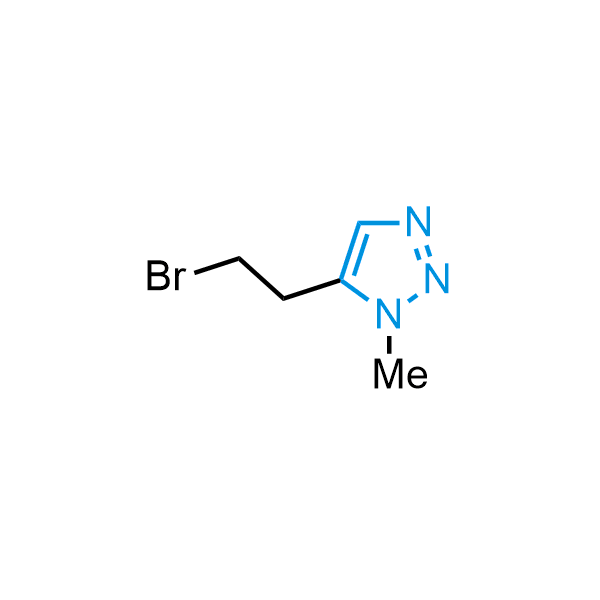
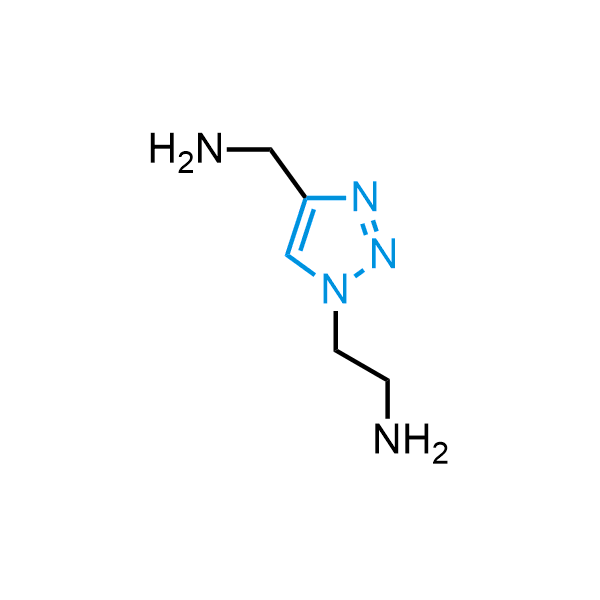
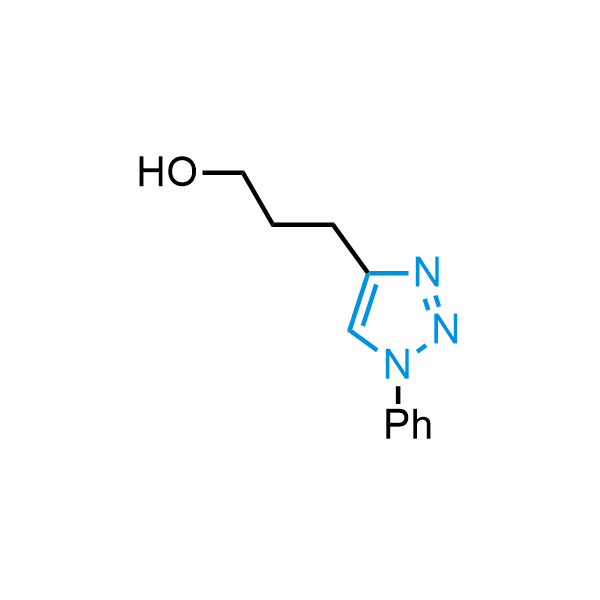
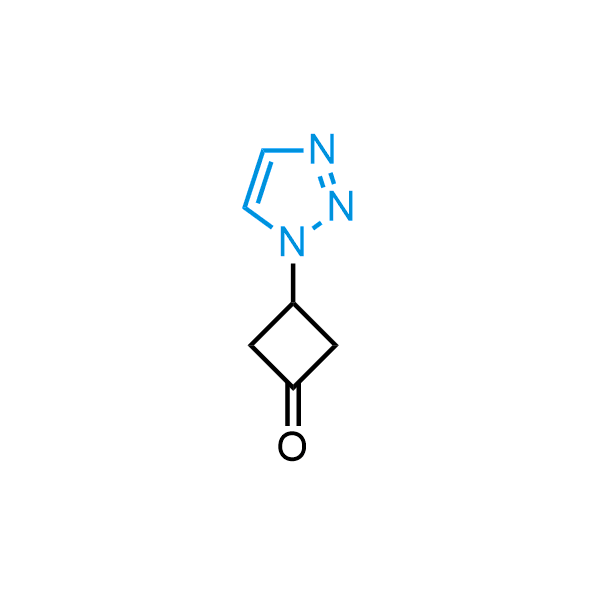
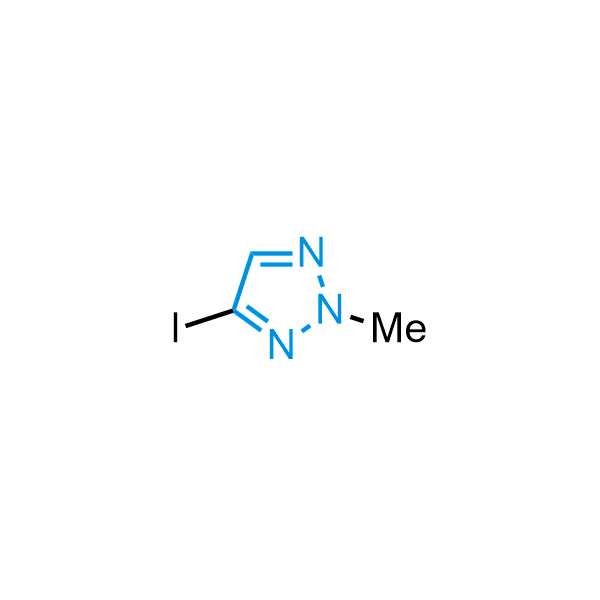
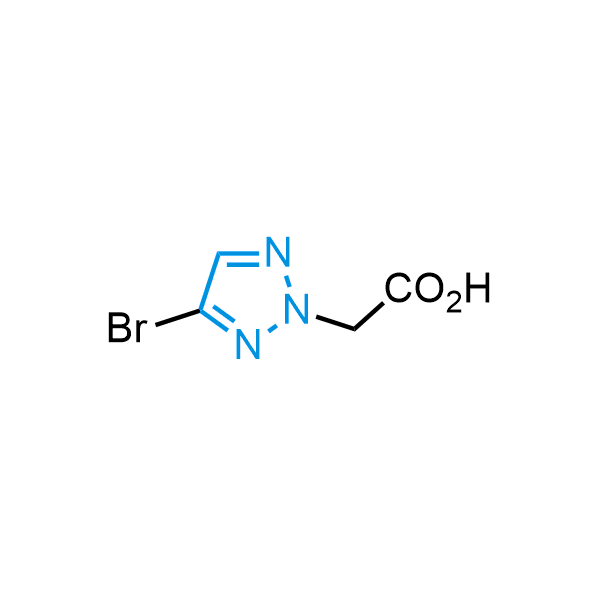
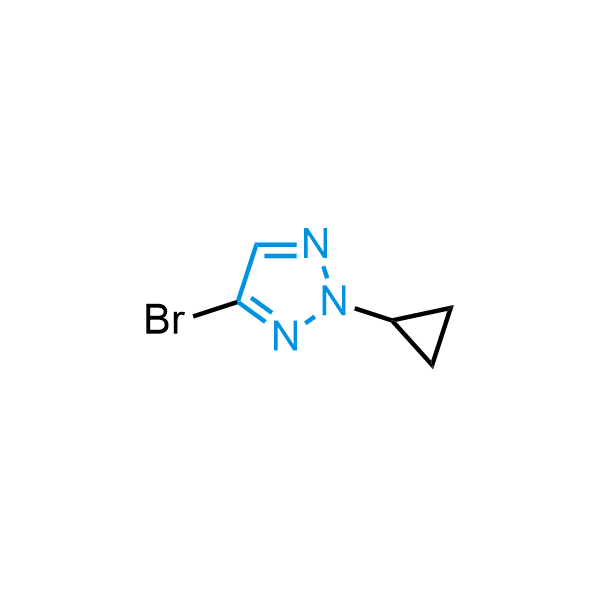
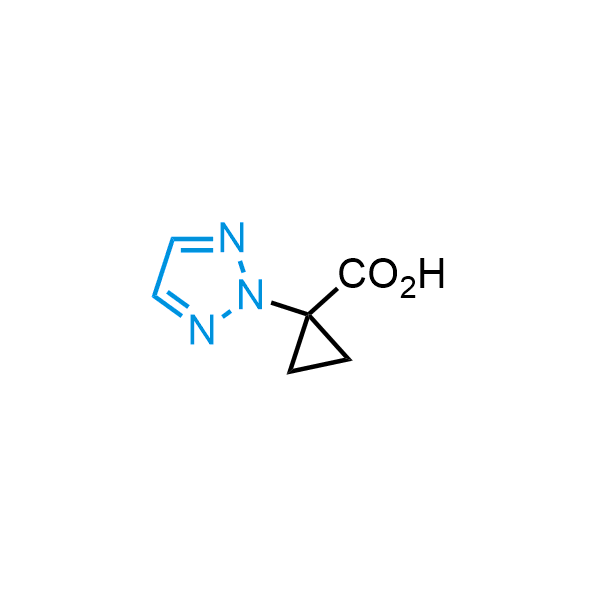
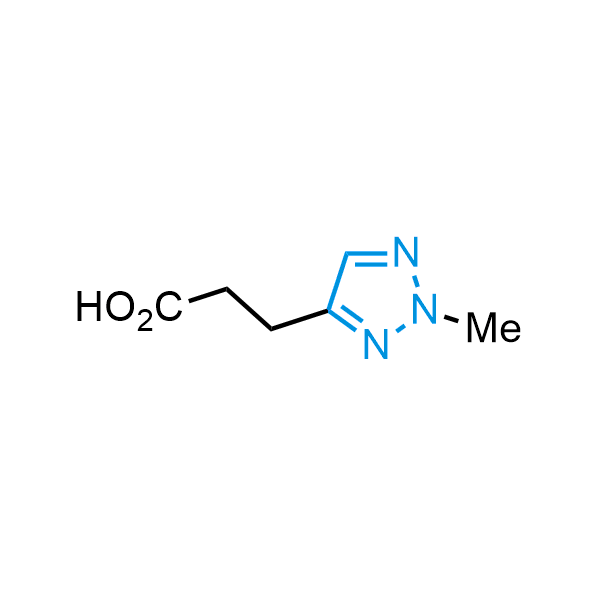
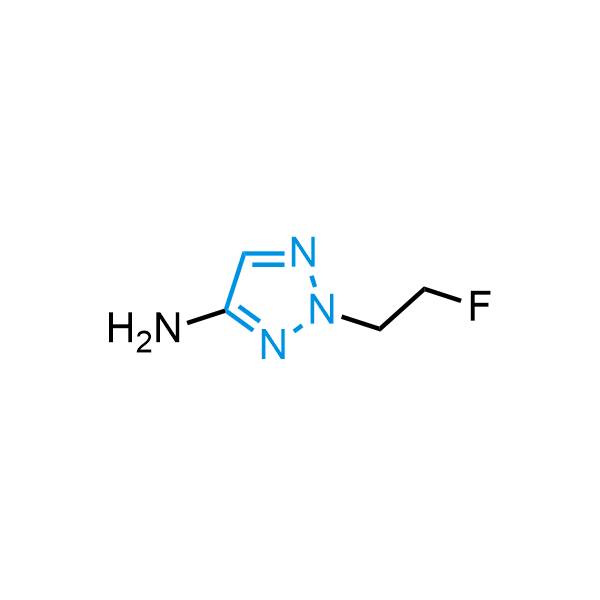
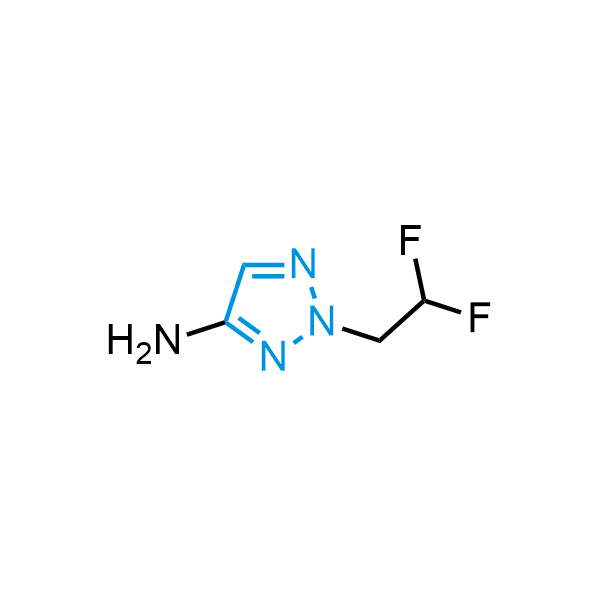
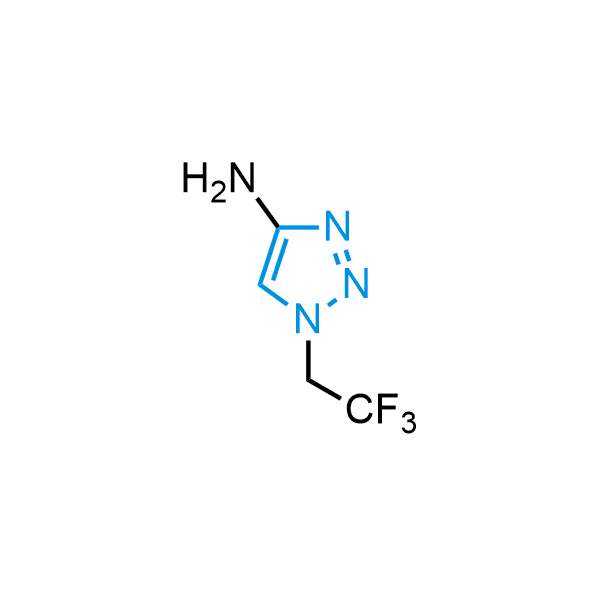
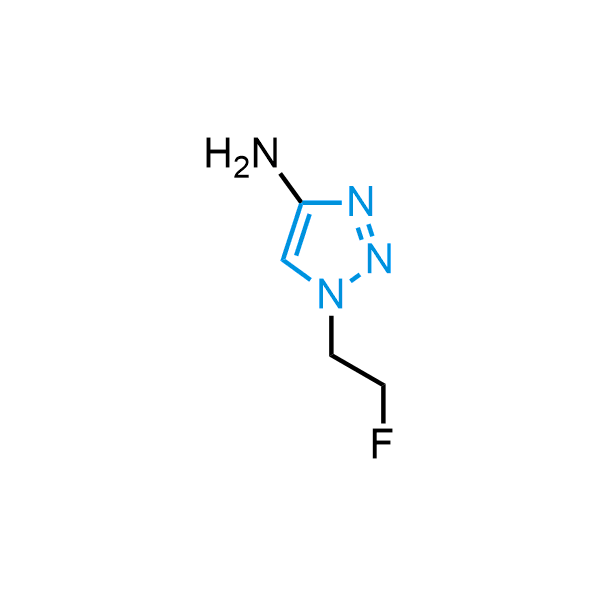
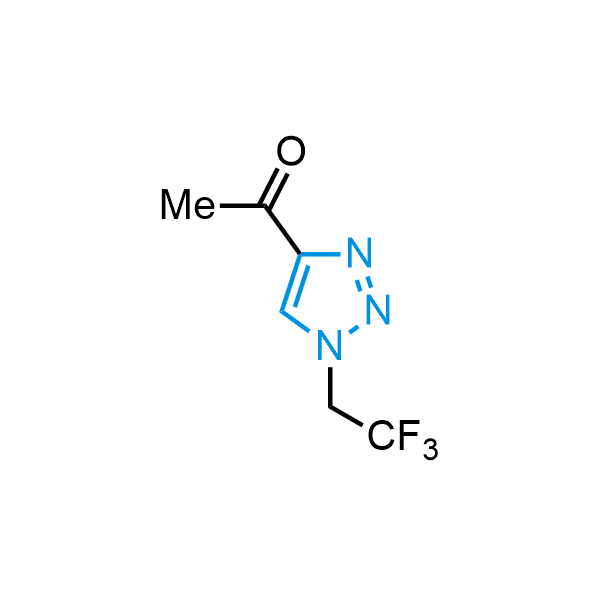
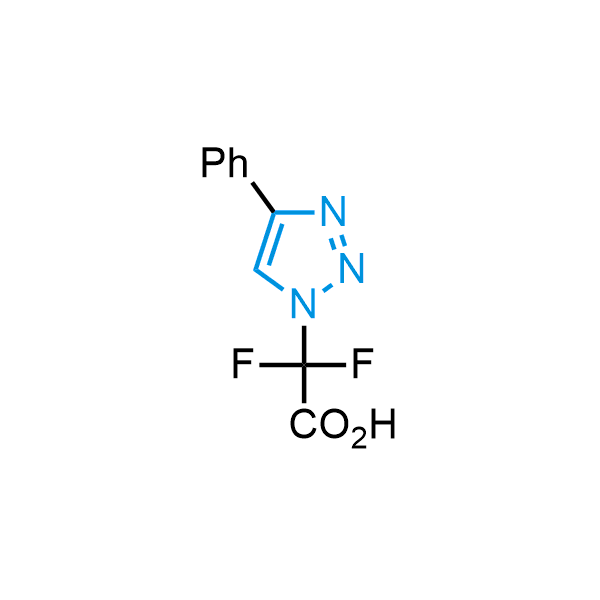
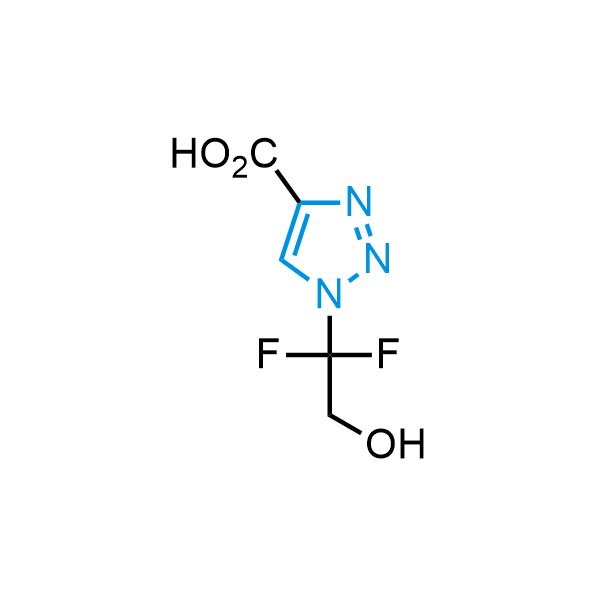
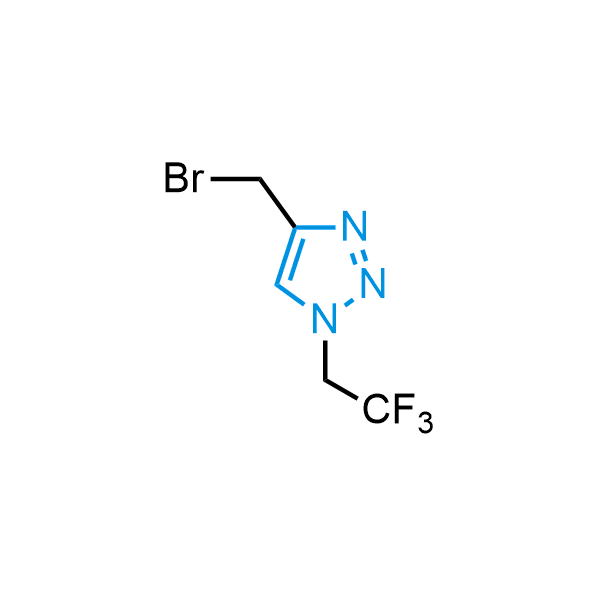
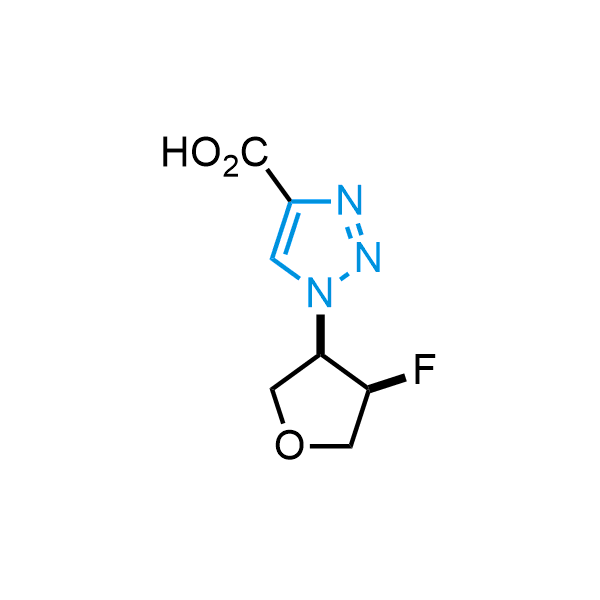
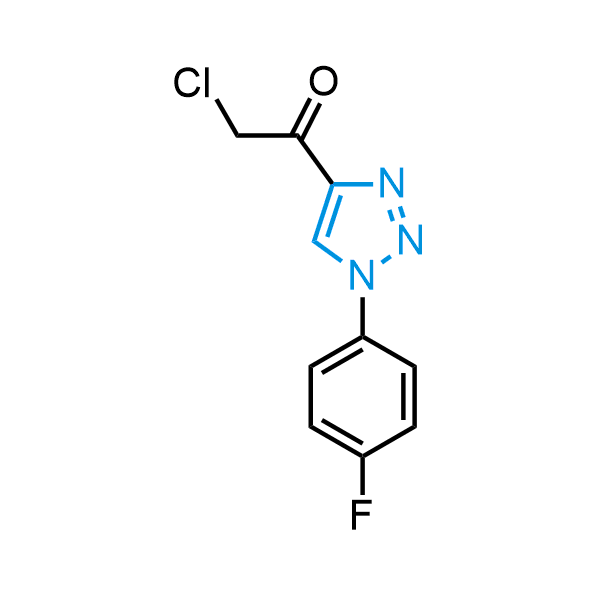
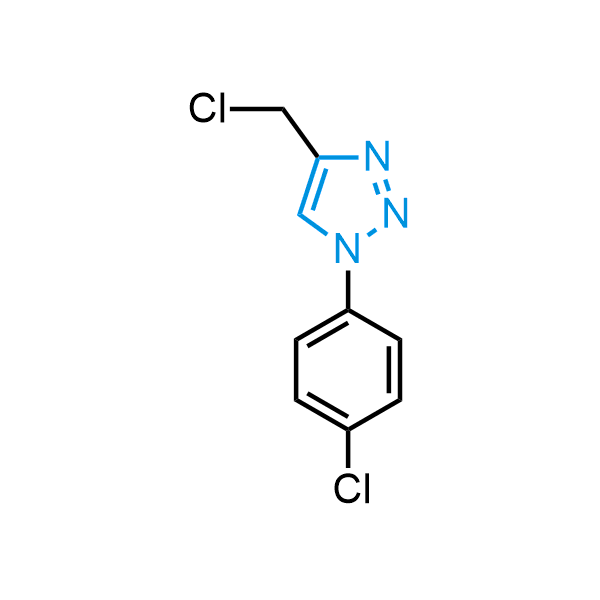
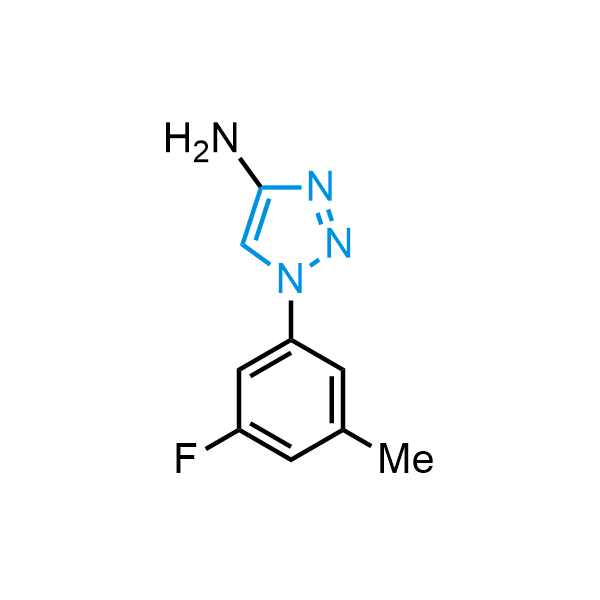
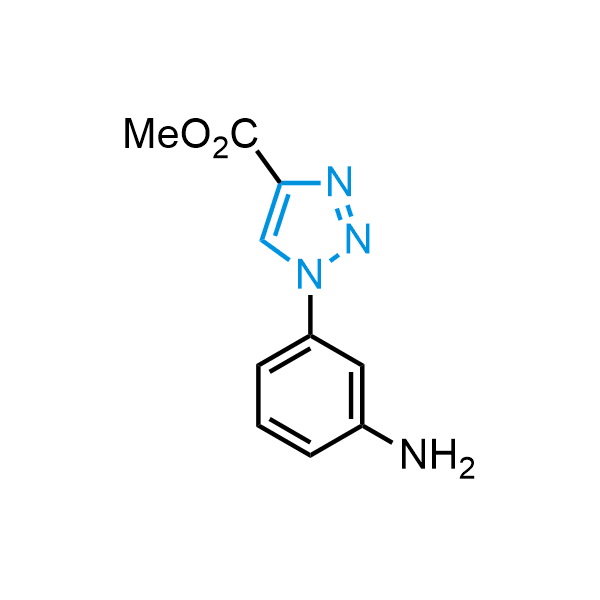
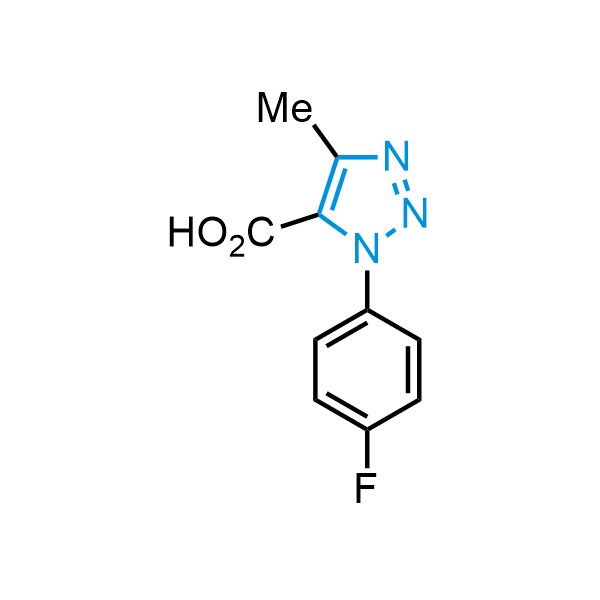
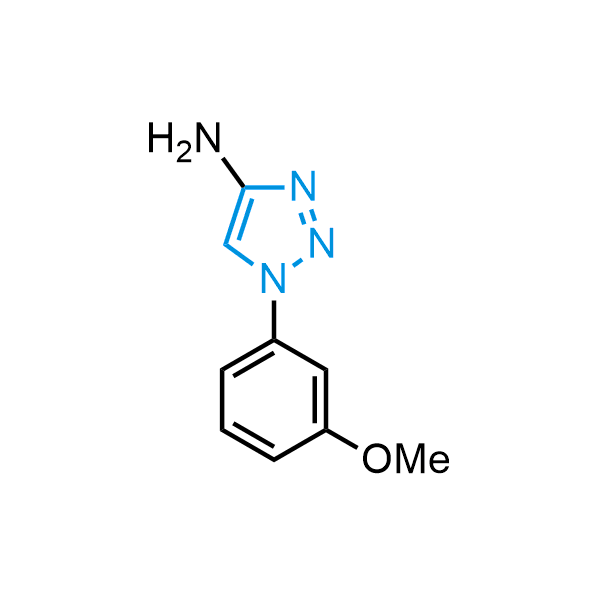
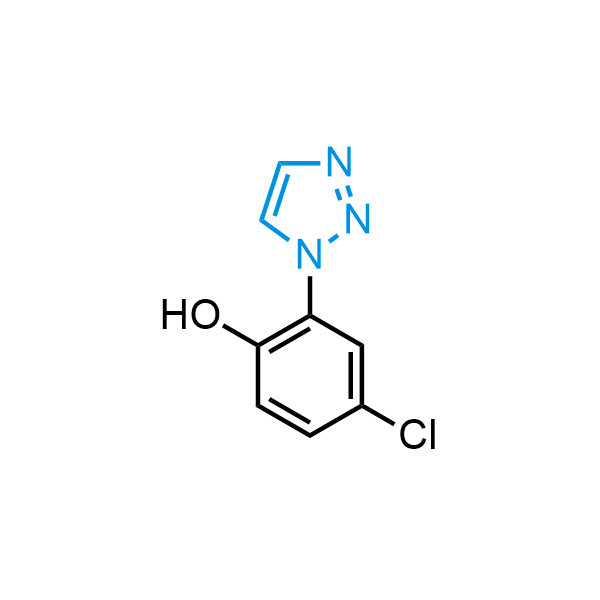
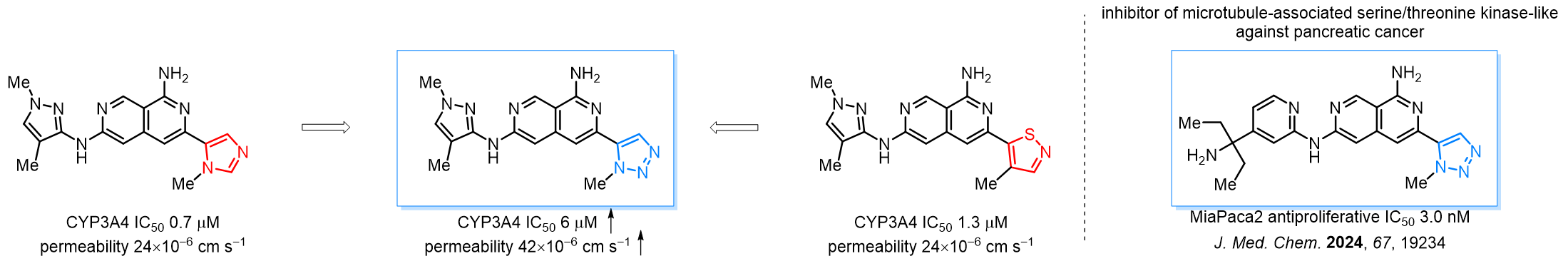
Triazoles are increasingly used in drug design due to their low lipophilicity, compact size, and weak basicity, making them excellent alternatives to other aromatic systems such as phenyl and azole rings. 1,2,3-Triazoles offer the additional benefit of poor iron ion coordination, reducing off-target inhibition of cytochrome P450. N-Alkylation is key to preventing tautomerization and defining the triazole fragment's dipole moment. Explore our collection of N-alkyl/aryl 1,2,3-triazole building blocks for your research.
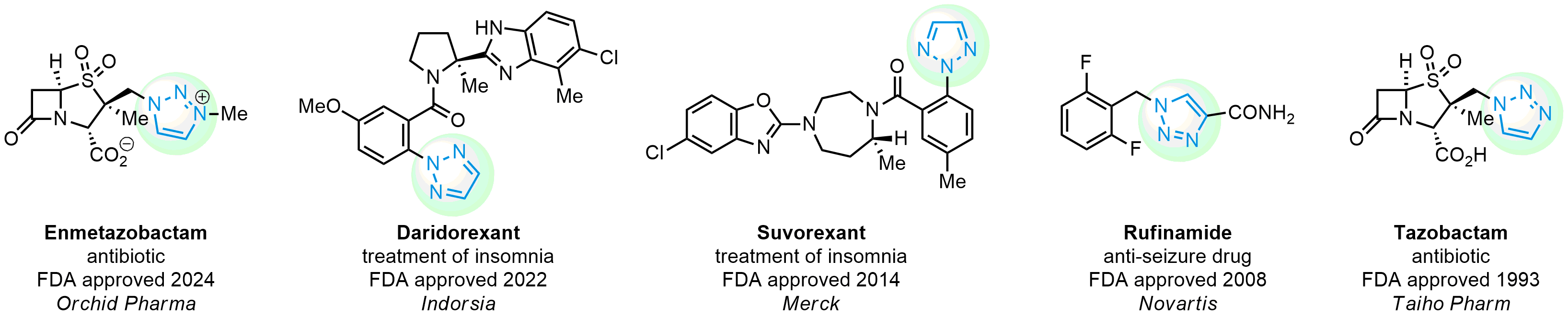
Case study
Download SD file
Download PDF file
We offer
Over 1,000 N-alkyl/aryl 1,2,3-triazoles from stock on 5-10 gram scale.