- Can modulate properties of peptides when introduced into their molecules
- Very promising building blocks for drug discovery (two most common functional groups!)
- Over 20 years of research at Enamine
- Design approaches: conformational restriction, fluorination, isosteric replacements
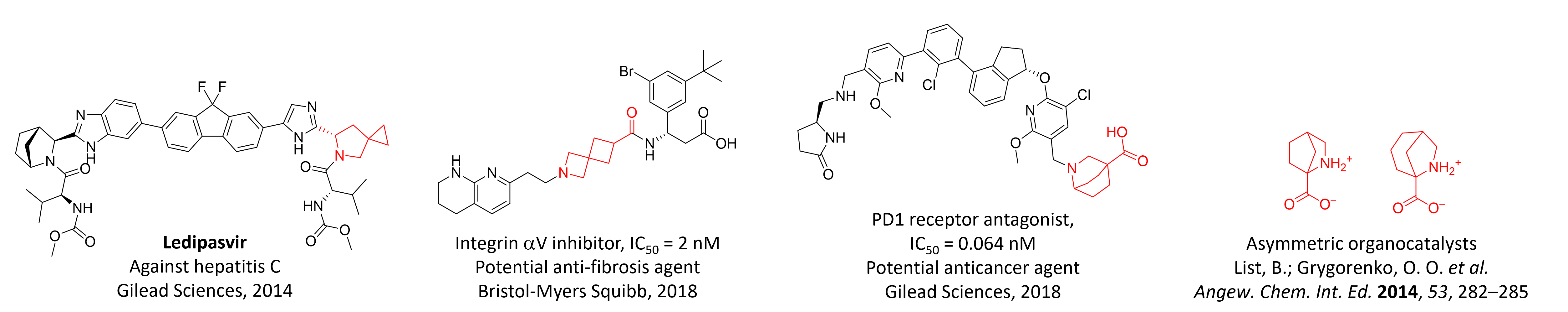
Amino acids, including unnatural derivatives, play a pivotal role in medicinal chemistry due to their ability to modulate the properties of peptides and serve as versatile building blocks for drug design. Their intrinsic combination of amino and carboxylic functional groups makes them highly valuable in therapeutic development. At Enamine, over 20 years of research have been devoted to this field, with a focus on synthetic strategies such as conformational restriction, fluorination, and isosteric replacements. These modifications enhance the functionality and stability of peptides and small molecules, providing innovative solutions for drug discovery. Here we highlight examples of amino acids synthesized at Enamine and their successful integration into drug candidates, showcasing their potential to drive advancements in medicinal chemistry.
https://doi.org/10.1002/anie.201306037
Spirocyclic Glutamate Analogs
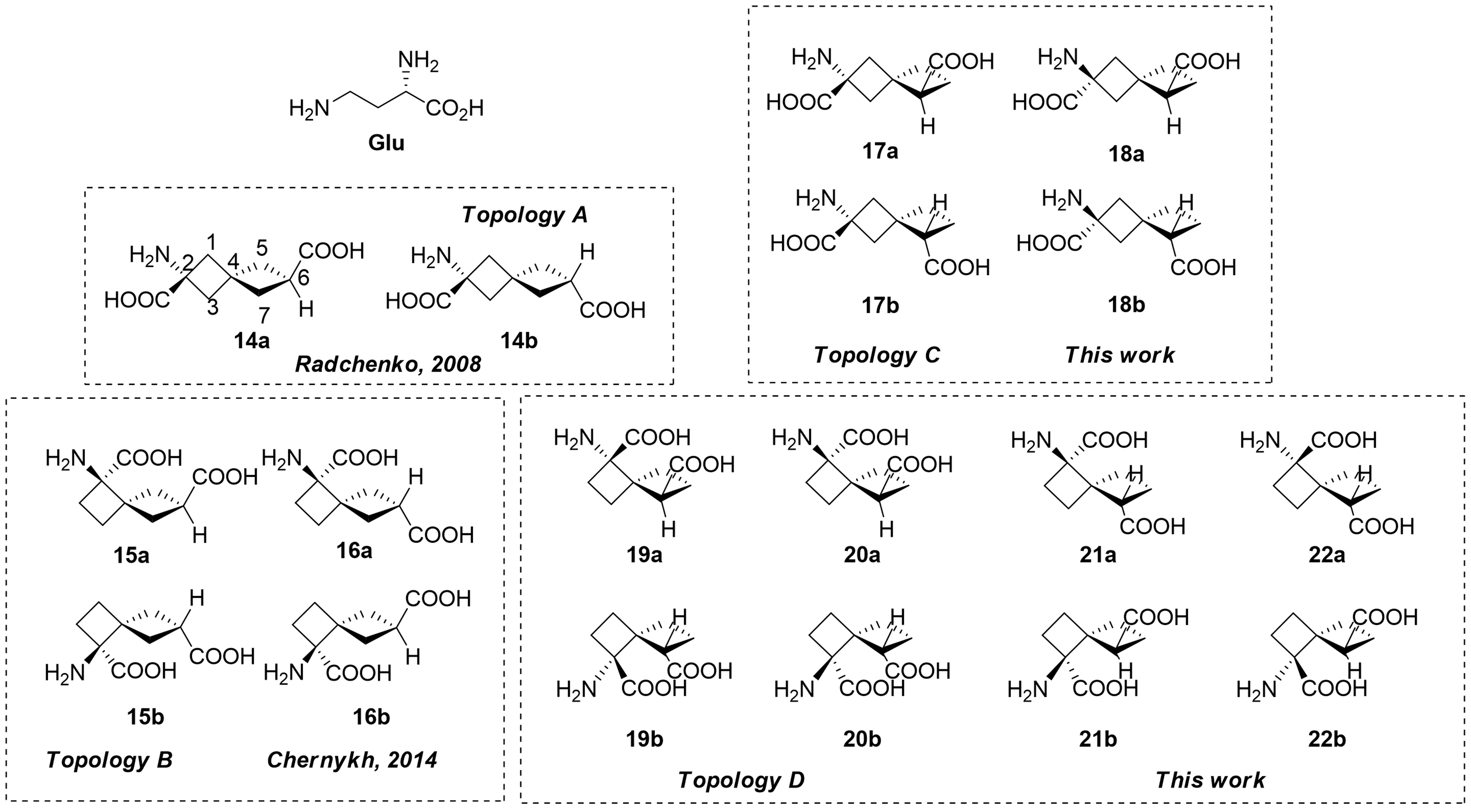
Chernykh, A. V.; Chernykh, A. V.; Radchenko, D. S.; Chheda, P. R.; Rusanov, E. B.; Grygorenko, O. O.; Spies, M. A.; Volochnyuk, D. M.; Komarov, I. V. A Stereochemical Journey around Spirocyclic Glutamic Acid Analogs. Organic & Biomolecular Chemistry, 2022, 20, 3183–3200. https://doi.org/10.1039/d2ob00146b.
Abstract: Spirocyclic glutamate analogs represent a significant focus of amino acid research at Enamine. This project, initiated in the mid-2000s, involved synthesizing spiro[3,3]heptane derivatives and culminated in a complete stereolibrary by 2022. Utilizing advanced synthetic strategies such as the Strecker reaction with Ellman’s sulfinamide and [2+2] cycloaddition, researchers achieved 16 of the 18 theoretically possible isomers. Notably, the Meinwald oxirane rearrangement played a key role in obtaining cyclobutanones for Topology D. The challenges of intramolecular lactam formation limited access to certain isomers, underscoring the complexity and innovation in this field. These analogs showcase the potential of spirocyclic structures in drug discovery.
Spirocyclic C6/C7 Amino Acids
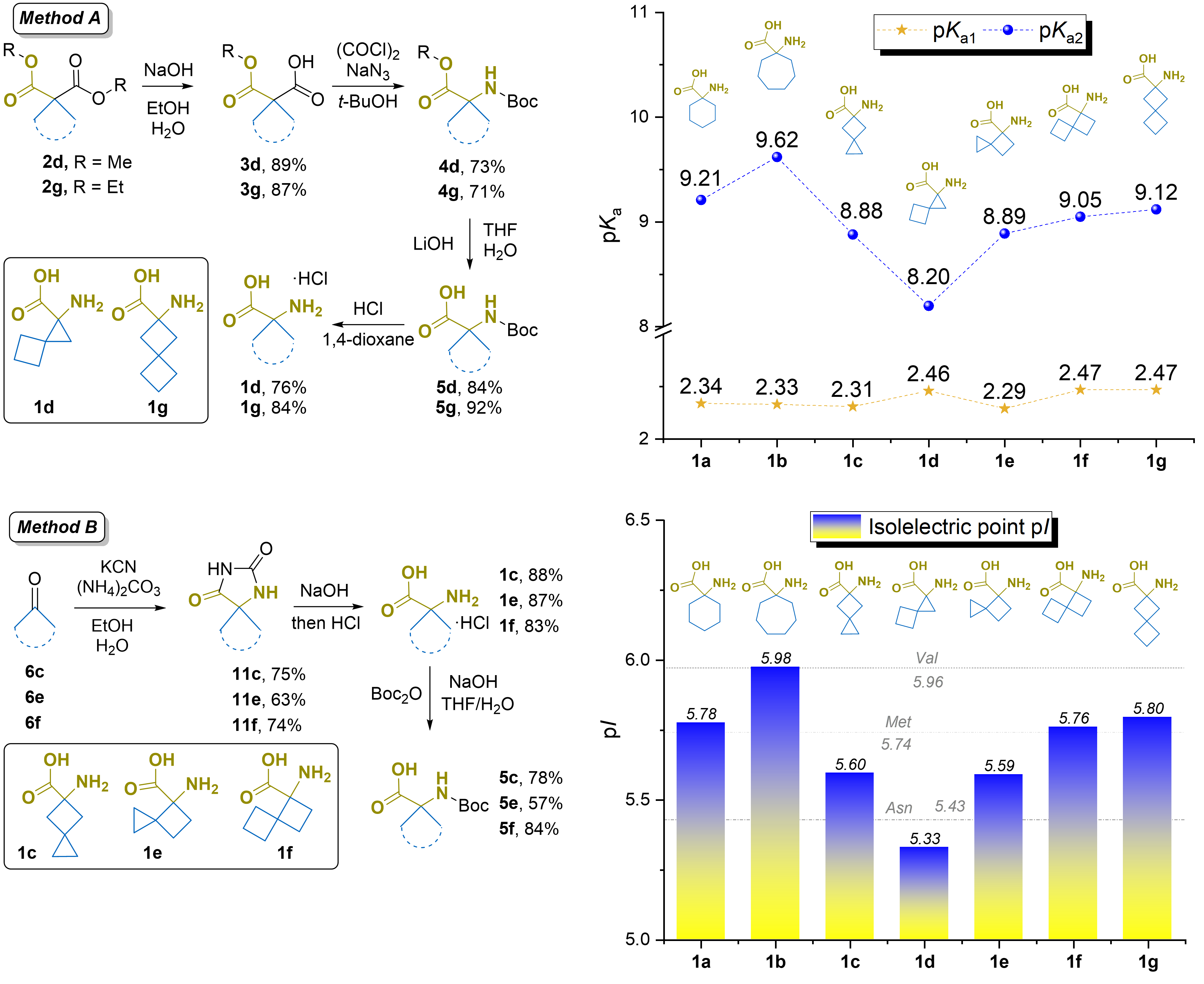
Malashchuk, A.; Chernykh, A. V.; Liashuk, O. S.; Hurbanov, R.; Lomaka, M.; Tkachuk, H.; Granat, D.; Grygorenko, O. O. Spiro[2.3]Hexane‐ and Spiro[3.3]Heptane‐derived α‐Amino Acids: Synthesis and Isoelectric Point Evaluation. ChemistrySelect, 2024, 9. https://doi.org/10.1002/slct.202402108.
Abstract: We explored the synthesis of spirocyclic amino acids with 6- or 7-membered spirocyclic systems using two primary approaches: the Curtius rearrangement of monoesters, yielding two compounds, and hydantoin formation followed by hydrolysis to produce target amino acids. Isoelectric points (pI) and pKa values of these spirocyclic derivatives were evaluated, revealing only slight reductions compared to monocyclic analogs, particularly for cyclopropane derivatives. These spirocyclic amino acids demonstrated acid-base properties that mimic methionine or asparagine, with changes mainly affecting the amino group’s basicity rather than the carboxylate function. This highlights their potential as versatile tools in medicinal chemistry.
Spiro[2.3]hexane-derived Peptidomimetics
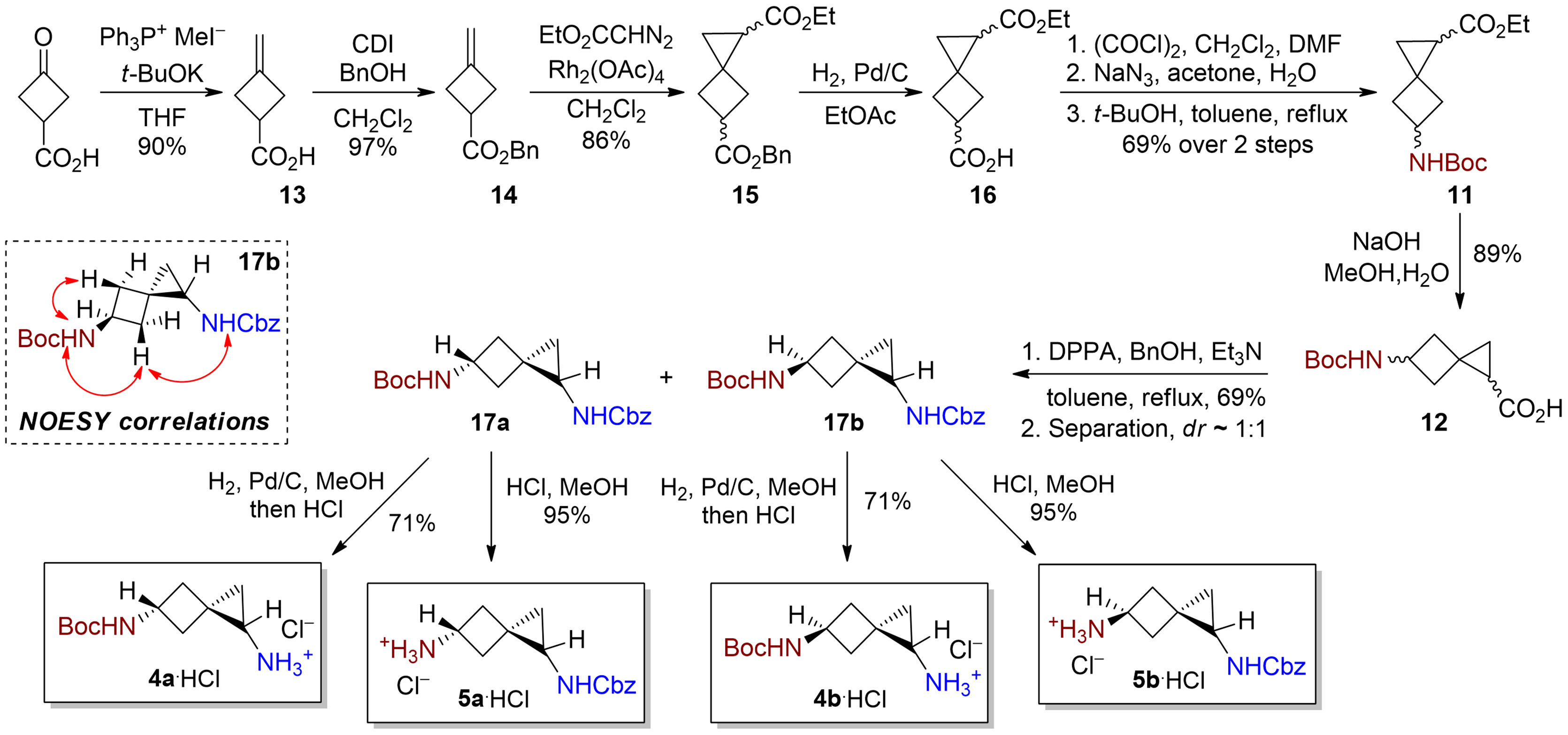
Malashchuk, A.; Chernykh, A. V.; Perebyinis, M. Y.; Komarov, I. V.; Grygorenko, O. O. Eur. J. Org. Chem. 2021, 6577–6586.
Abstract: Spiroheptane derivatives were synthesized via cyclopropanation of cyclobutane, resulting in diastereomeric mixtures. While separation proved challenging during intermediate steps, Curtius rearrangement enabled the production of orthogonally protected diamines separable by chromatography at gram scale. These monoprotected diamine building blocks are valuable in early drug discovery. X-ray diffraction revealed interesting structural properties: one isomer mimicked β-turns in peptides, while another exhibited a β-sheet-like structure. These findings highlight the potential of spiroheptane derivatives in the design of peptidomimetics for medicinal applications.
Isonipecotic acid analogs: azabicyclo[3.1.1]heptanes
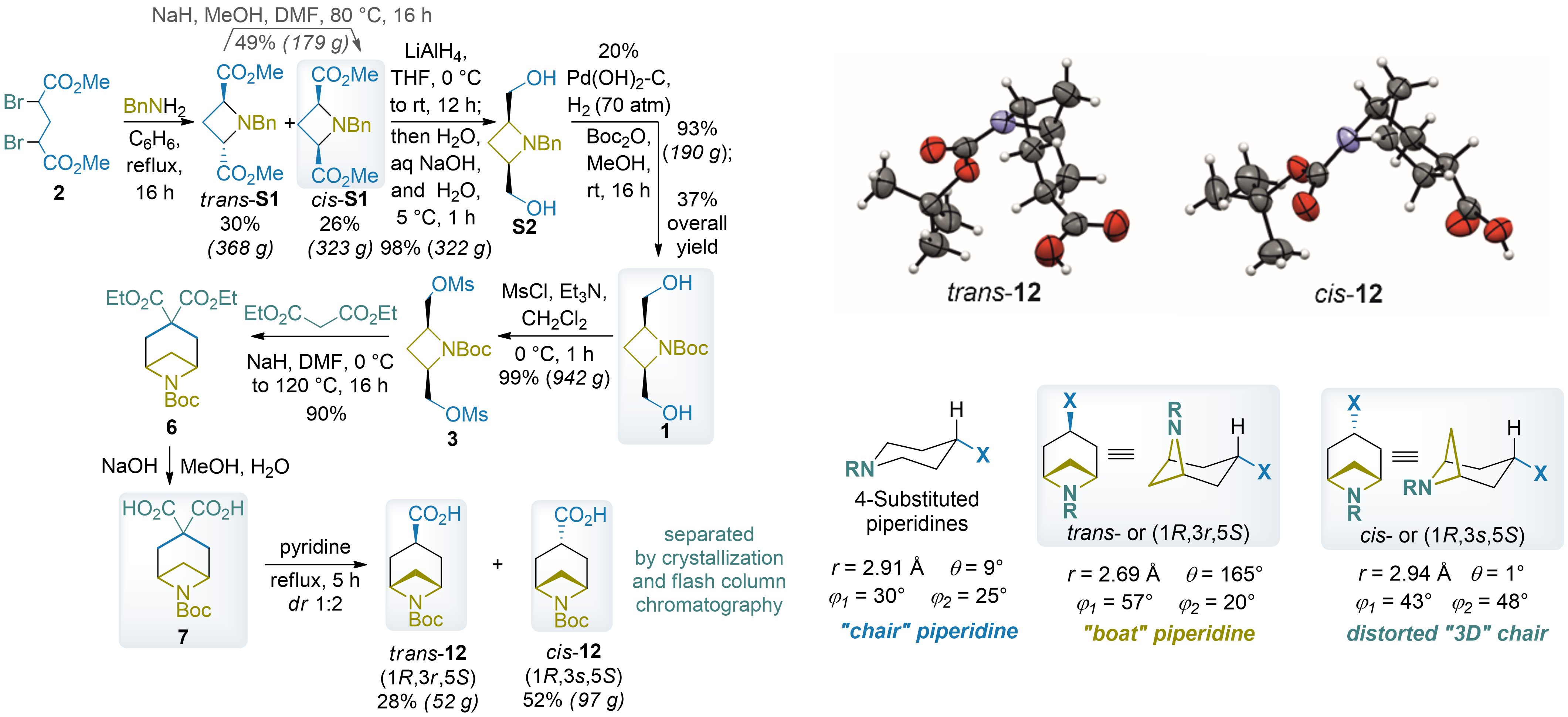
Chernykh, A. V.; Vashchenko, B. V.; Shishkina, S. V.; Volochnyuk, D. M.; Grygorenko, O. O. 3-Substituted 6-azabicyclo[3.1.1]heptanes: nonclassical piperidine isosteres for drug discovery. J. Org. Chem. 2024, 89(15), 10440–10450. DOI: 10.1021/acs.joc.4c00326
Abstract: We expanded our research to include bridged and fused bicyclic amino acids, synthesizing isonipecotic acid analogs through a key sequence involving double alkylation of malonate with azetidine-derived bis-mesylate. Subsequent decarboxylation yielded isomeric monoprotected isonipecotic acids, which were efficiently separated on a large scale. X-ray diffraction revealed that these compounds can mimic piperidine rings in distinct conformations. One isomer adopts a distorted 3D chair conformation, while the other fixes the piperidine in a rare and unusual boat conformation, underscoring their unique structural properties and potential applications in medicinal chemistry.
Housane-derived GABA analogs
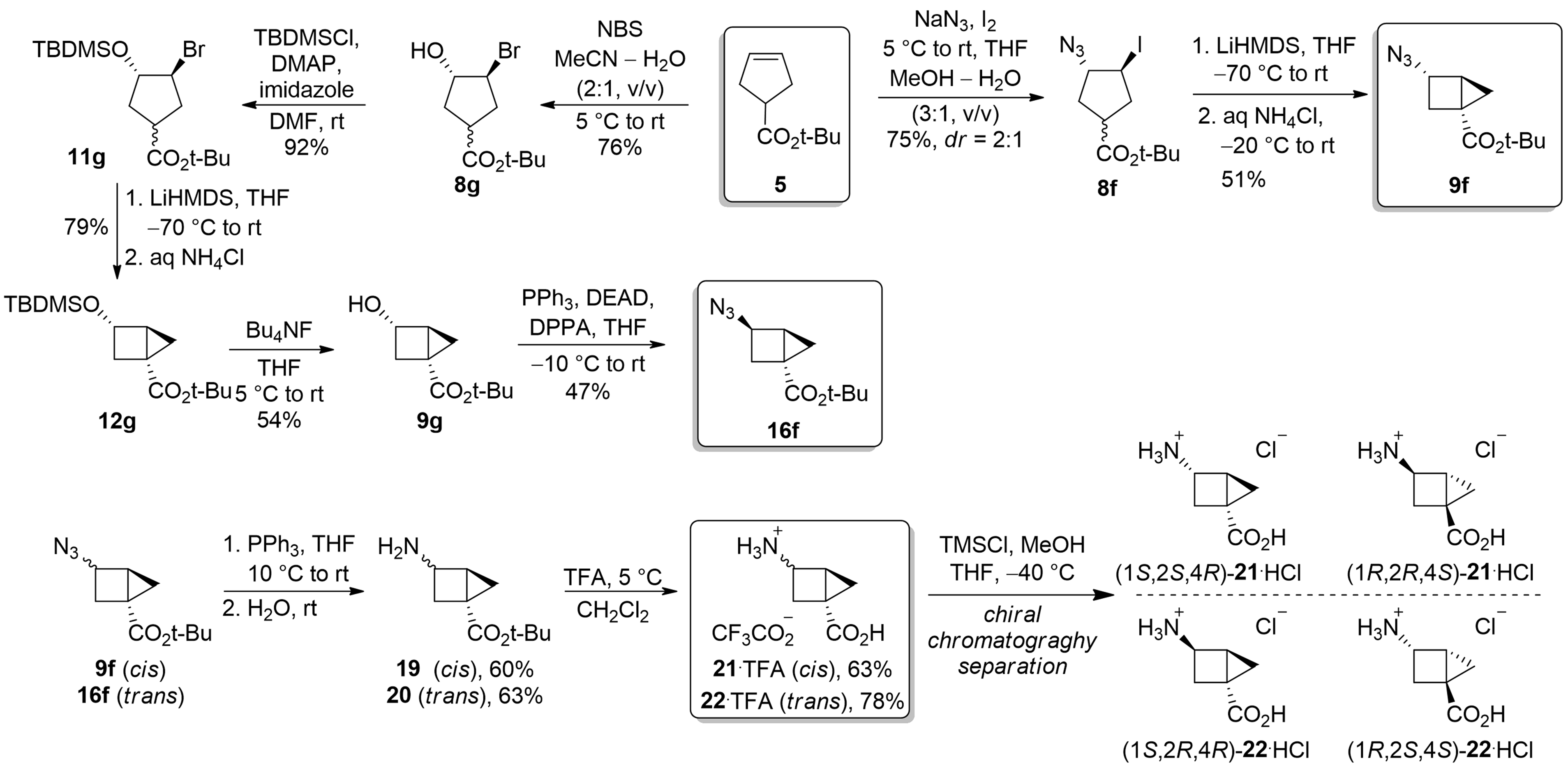
Semeno, V. V.; Vasylchenko, V. O.; Vashchenko, B. V.; Lutsenko, D. O.; Iminov, R. T.; Volovenko, O. B.; Grygorenko, O. O. Building the Housane: Diastereoselective Synthesis and Characterization of Bicyclo[2.1.0]Pentane Carboxylic Acids. The Journal of Organic Chemistry, 2019, 85, 2321–2337. https://doi.org/10.1021/acs.joc.9b03044.
Absract: Gamma-amino acids were synthesized using a bicyclo[2,1,0]pentane scaffold via iodo-azidation and intramolecular enolate alkylation to form a bicyclic system. Alternative steps, including bromo-hydroxylation and Mitsunobu reaction, allowed access to another diastereomer. Chromatographic separation of both diastereomers yielded four stereoisomers at subgram scale.
In parallel, a tetrafluorinated GABA analog was synthesized from a commercially available diol through functional group transformations, including monoprotection, amino group introduction, and selective oxidation. This analog exhibited altered pKa values, highlighting fluorination effects, and conformational analysis revealed similarities to GABA conformers selective for specific receptor subtypes. These findings emphasize their utility in receptor studies and medicinal chemistry.
Trifluoromethyl-substituted α/β-Prolines
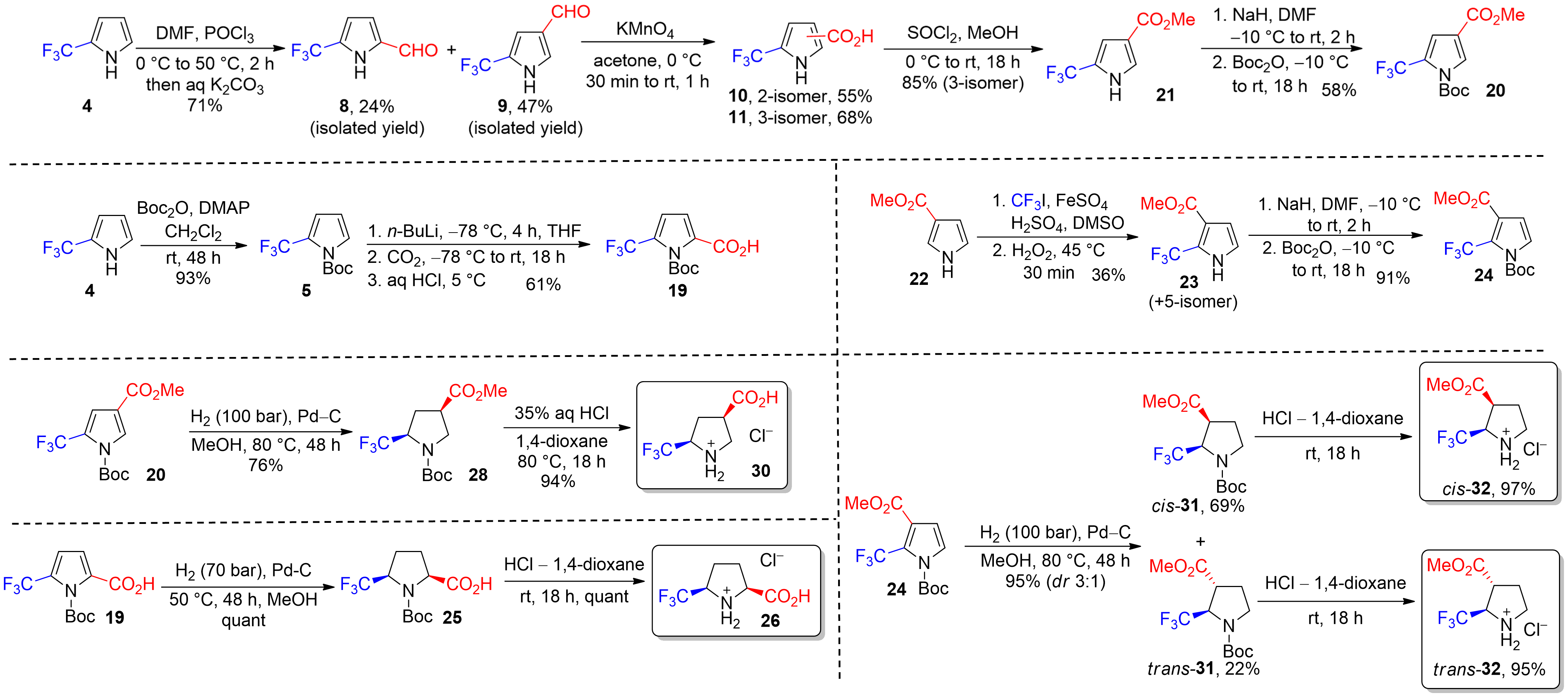
Hys, V. Y.; Shevchuk, O. I.; Vashchenko, B. V.; Karpenko, O. V.; Gorlova, A. O.; Grygorenko, O. O. Eur. J. Org. Chem. 2020, 3896–3905.
Abstract: A series of alpha- and beta-prolines containing CF3 groups were synthesized starting from pyrrole derivatives. Key steps included hydrogenation and functional group transformations. For 2,5-substituted prolines, lithiation and carboxylation of organometallic intermediates provided carboxylic acids, while 2,3-disubstituted prolines were synthesized through trifluoromethylation of pyrrole derivatives. Hydrogenation and deprotection steps yielded the target prolines, though the final step for 2,3-disubstituted prolines was not fully diastereoselective. The resulting compounds were produced in good yields and offer valuable building blocks for medicinal chemistry applications.
CF3/C2F5-substituted Prolines

Chernykh, A. V.; Aloshyn, D.; Kuchkovska, Yu. O.; Daniliuc, C. G.; Tolmachova, N. A.; Kondratov, I. S.; Zozulya, S.; Grygorenko, O. O.; Haufe, G. Org. Biomol. Chem. 2022, 20, 9337–9350.
Abstract: Using fluorinated proline analogs, enantiopure compounds were synthesized through the separation of diastereomeric amides, followed by hydrolysis to form model dipeptides. These were tested for plasma stability to evaluate the impact of fluorinated substituents. Results showed that while the trans-fluorinated S-proline derivative exhibited reduced stability, other isomers displayed significant resistance to enzymatic hydrolysis, highlighting their potential in designing stable bioactive compounds.
Fluorination + Conformational Restriction
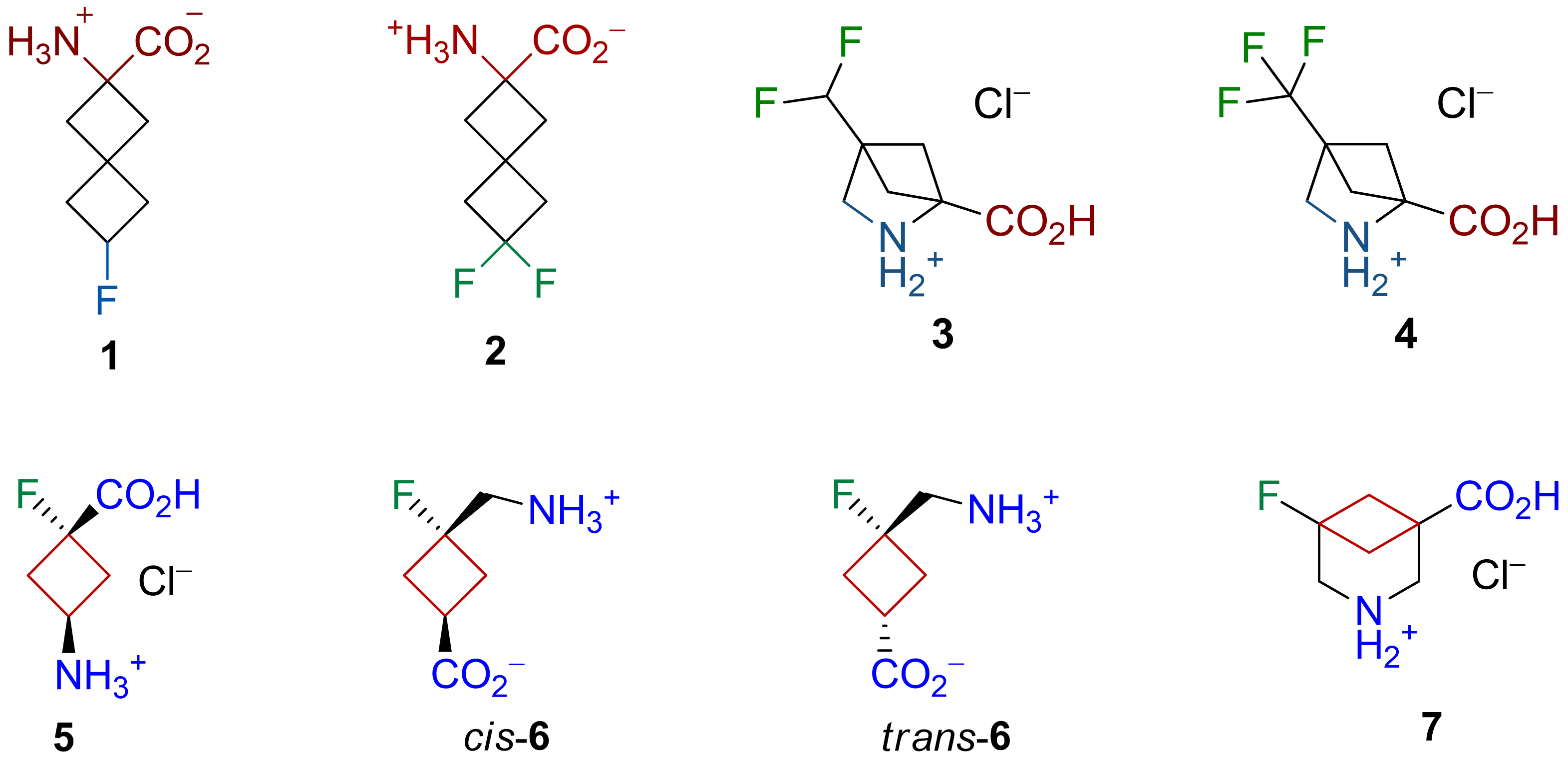
Malashchuk, A.; Chernykh, A. V.; Dobrydnev, A. V.; Grygorenko, O. O. Eur. J. Org. Chem. 2021, 4897–4910.
Olifir, O. S.; Chernykh, A. V.; Dobrydnev, A. V.; Grygorenko, O. O.; Moroz, Yu. S.; Voitenko, Z. V.; Radchenko, D. S. Eur. J. Org. Chem. 2021, 6541–6550.
Homon, A. A.; Hryshchuk, O. V.; Mykhailenko, O. V.; Vashchenko, B. V.; Melnykov, K. P.; Michurin, O.; Daniliuk, C. G.; Gerus, I. I.; Kovtunenko, V. O.; Kondratov, I.; Grygorenko, O. O. Eur. J. Org. Chem. 2021, 6587–6597.
Feskov, I. O.; Golub, B. O.; Vashchenko, B. V.; Levterov, V. V.; Kondratov, I. S.; Grygorenko, O. O.; Haufe, G. Eur. J. Org. Chem. 2020, 4755–4767.
Abstract: We combined fluorination with conformational restriction to synthesize unique amino acid building blocks for drug discovery. This approach yielded spirocyclic compounds, difluoro- and trifluoromethyl methanoproline derivatives, monofluorinated γ-amino acid analogs, and methanonipecotic derivatives with fluorine atoms. These structures offer potential for developing bioactive molecules with enhanced properties. Detailed synthetic methods are available in recent publications for further reference.
Pseudonatural products in Enamine Collection
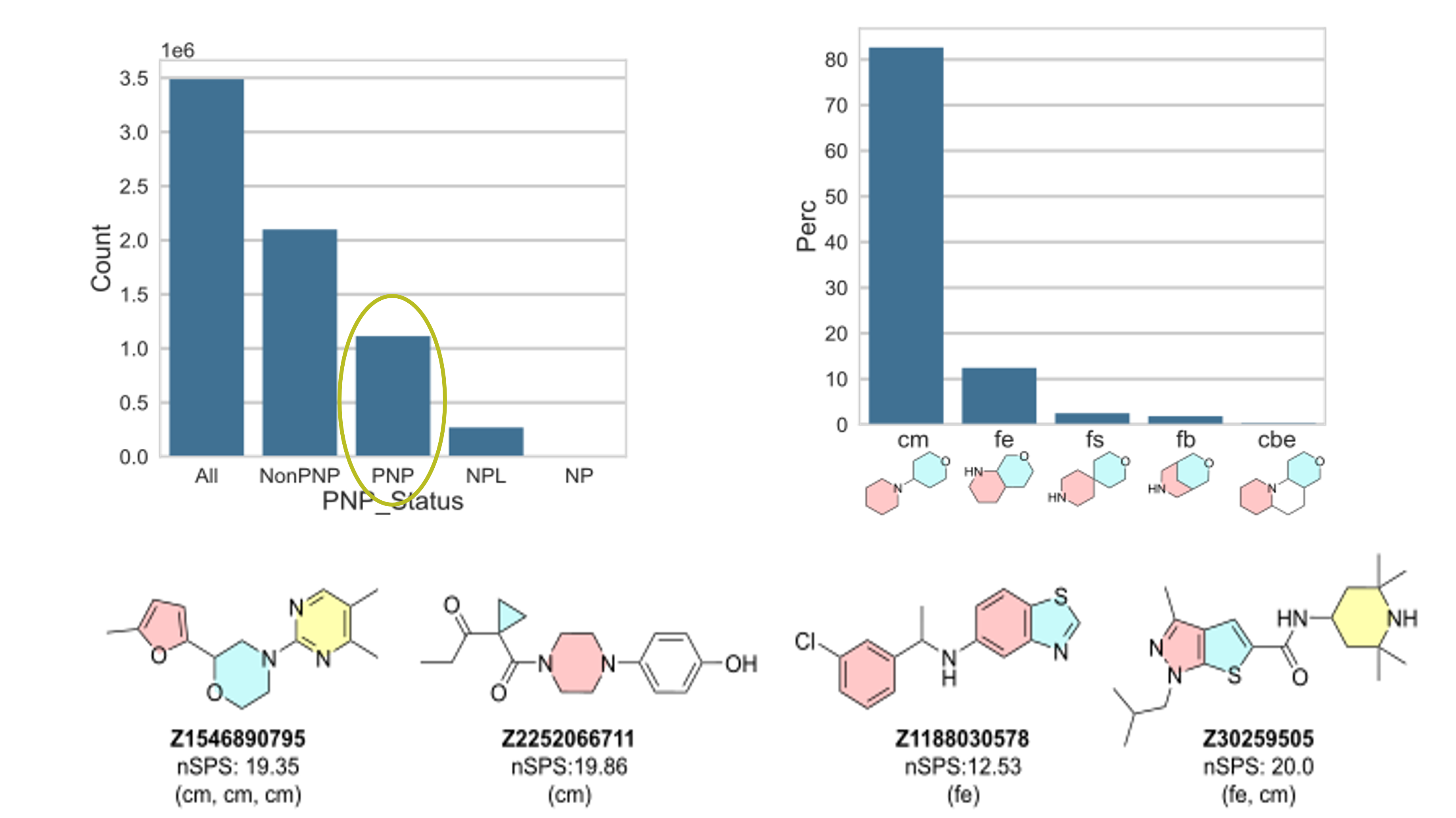
Pahl, A.; Grygorenko, O. O.; Kondratov, I. S.; Waldmann, H. Identification of Readily Available Pseudo-Natural Products. RSC Medicinal Chemistry, 2024, 15, 2709–2717. https://doi.org/10.1039/d4md00310a.
Abstract: Inspired by Professor Waldmann’s concept of pseudonatural products, we analyzed our stock collection (3.5 million compounds) and identified over 1 million pseudonatural products. These compounds are synthesized by combining fragments from natural product scaffolds to create novel molecules not found in nature. They show great promise for biological activity, as demonstrated by cell painting assays. Additionally, scaffold analysis, guided by ring system connections, revealed many biologically active compounds, some of which are highlighted in this slide.
