Formulation scale-up is a significant challenge in drug development. Often, the initial research and development phases focus on optimizing the formulation at a milligram scale without fully addressing the complexities associated with scaling up for large-scale production. This oversight can lead to difficulty connected with transitioning from lab-scale to commercial manufacturing.
By focusing on this problem, Enamine chemists can effectively address the challenges of scaling up synthetic processes in cost-effective manner, leading to more successful transitions from lab-scale to commercial production.
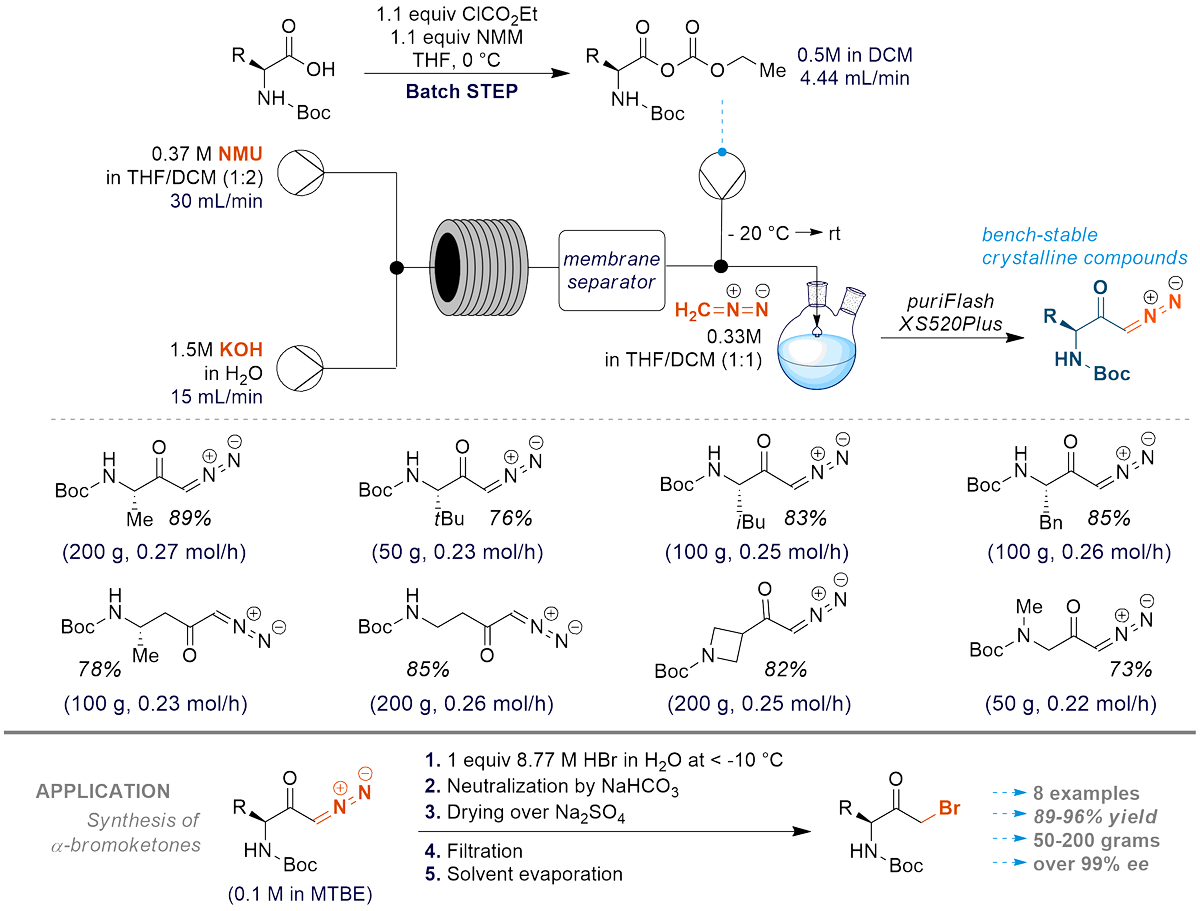
Shelf-stable amino acids derived diazoketones
Pendiukh, V. V.; Yakovleva, H. V.; Stadniy, I. A.; Pashenko, A. E.; Rusanov, E. B.; Grabovaya, N. V.; Kolotilov, S. V.; Rozhenko, A. B.; Ryabukhin, S. V.; Volochnyuk, D. M., Practical Synthetic Method for Amino Acid-Derived Diazoketones Shelf-Stable Reagents for Organic Synthesis. Org. Process Res. Dev. 2024, 28 (1), 165-176. https://doi.org/10.1021/acs.oprd.3c00230
Abstract: A novel approach for a safe generation of the diazomethane-containing organic solution has been proposed using a serial flow reactor. The productivity of the diazomethane generation has reached 0.45 mol/h. Several chiral diazo-ketones have been prepared in quantities up to 150 g through the reaction of diazomethane with mixed anhydrides, generated in situ by treatment of Boc-protected α-amino-acids with ethyl chloroformate. Synthesized diazoketones are crystalline substances stable up to 110-142 °C and can easily be purified on silica gel and stored for a long term. The overall stability and availability on a large scale allow for the consideration of such diazoketones as promising bench-stable regents for the broad scope of applications. The quantum chemical studies show that the stability of diazoketones relates to the conjugation of the carbonyl group with diazo moiety. As an example of applicability: the reaction of diazoketones with concentrated HBr gives corresponding pure chiral α-bromoketones without additional purification.
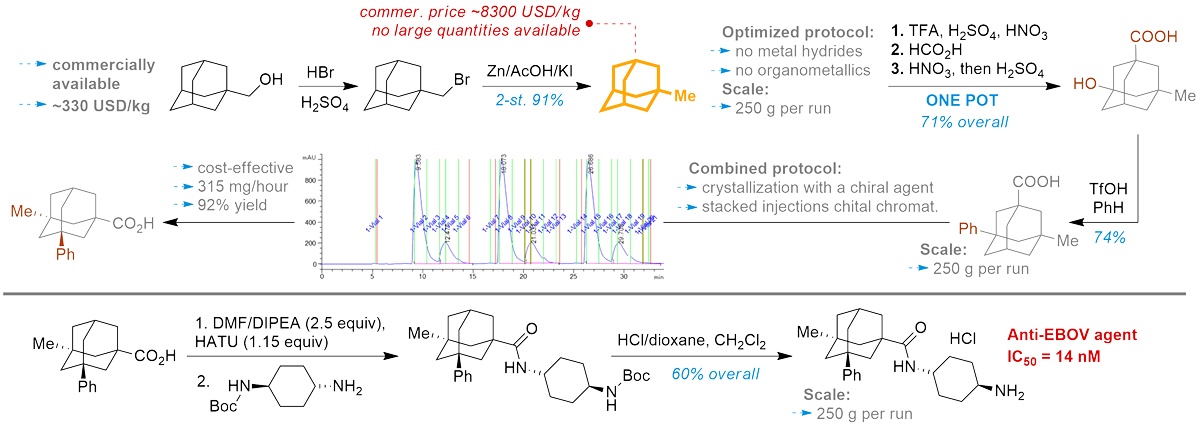
Rare cage chemotype
Pashenko, A. E.; Gaidai, A.; Hryhoriev, N.; Volovenko, O.; Levandovskiy, I.; Maksymenko, O.; Volochnyuk, D. M.; Ryabukhin, S. V., Scale-Up Synthesis of 1-Methyladamantane and Its Functionalization as a Key Point for Promising Antiviral Agents. Org. Process Res. Dev. 2023, 27 (3), 477-487. https://doi.org/10.1021/acs.oprd.2c00305
Abstract. Methyladamantane and its derivatives are rare chemotypes for industrial and even wide research use. The proposed scalable and inexpensive approach to such compounds opens the door to further extensive studies of biologically active derivatives based on this uncommon core. Optimized synthesis of the key precursor, the chiral (1S,3R,5R,7S)-3-methyl-5-phenyladamantane-1-carboxylic acid, showcases some significant innovations. The main one is very effective and cost-efficient stacked injections in preparative chiral chromatography and their potential for enantio-resolution of chiral cage compounds. Scaling the preparation of (1S,3R,5R,7S)-trans-N-(4-aminocyclohexyl)-3-methyl-5-phenyladamantane-1-carboxamide, which was recently shown to have notable activity against Ebola virus, to multigrams is an excellent example of our elaboration significance of. It may push forward further biological investigation of this promising antiviral agent.
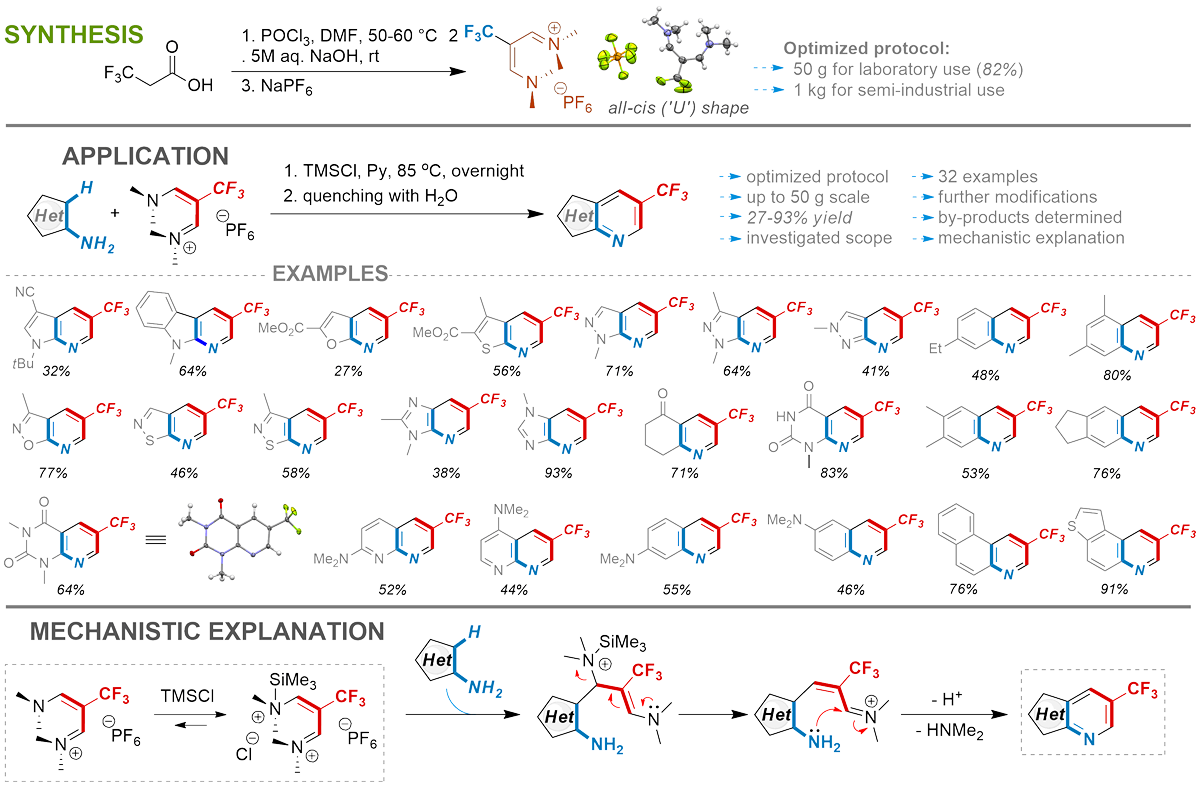
New application of known reagent
Mityuk, A. P.; Kiriakov, O. M.; Tiutiunnyk, V. V.; Lebed, P. S.; Grabchuk, G. P.; Rusanov, E. B.; Volochnyuk, D. M.; Ryabukhin, S. V. Trifluoromethyl Vinamidinium Salt as a Promising Precursor for Fused β-Trifluoromethyl Pyridines. J. Org. Chem. 2023, 88 (5), 2961-2972. https://doi.org/10.1021/acs.joc.2c02684
Abstract: Efficient chlorotrimethylsilane promoted synthetic protocol for the preparation of functionalized fused β-trifluoromethyl pyridines by cyclization of electron-rich aminoheterocycles or substituted anilines with trifluoromethyl vinamidinium salt was developed. The efficient and scalable approach for producing represented trifluoromethyl vinamidinium salt demonstrated huge prospects for further use. The structure specificities of the trifluoromethyl vinamidinium salt and their impact on the reaction progress were determined. The procedure's scope and alternative ways of the reaction were investigated. The possibility of increasing the reaction scale up to 50 grams and further modification of obtained products was shown. The mini-library of potential fragments for 19F-NMR-based FBDD was synthesized.
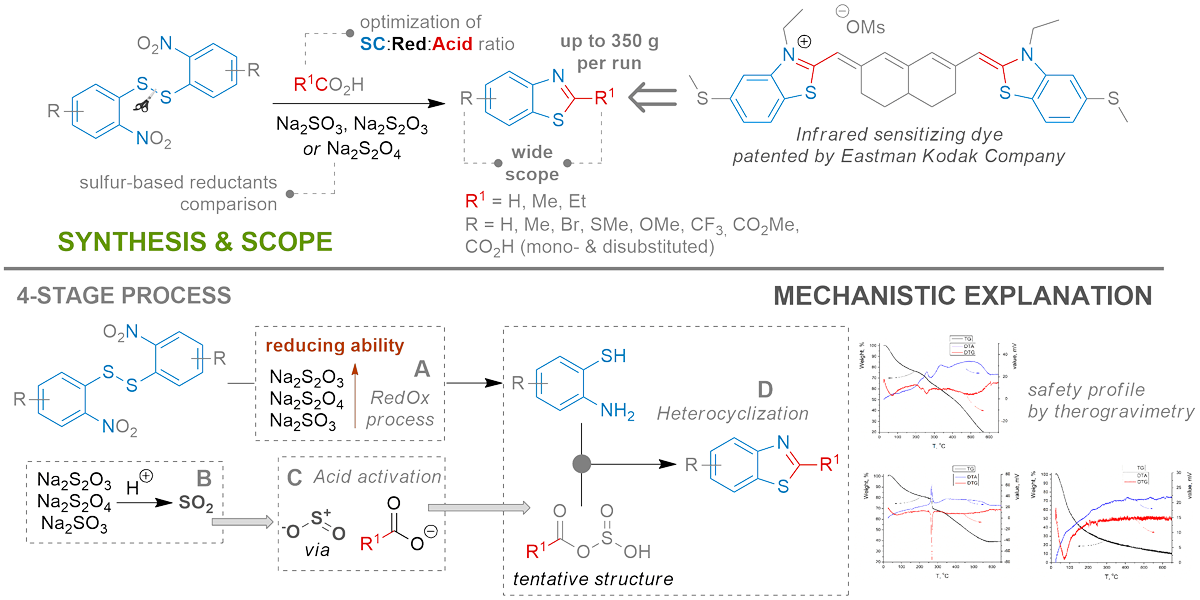
Conversion of rubbish into “gold” benzothiazoles
Puskov, V. O.; Babii, S. B.; Adamenko, I. V.; Melnychuk, S. O.; Druzhenko, T. V.; Lyapunov, A. Y.; Popov, S. V.; Volochnyuk, D. M.; Ryabukhin, S. V. Practical One-Pot Synthesis of 2-Alkyl-Substituted Benzothiazoles from Bis-(2-nitrophenyl)-disulfides. Org. Process Res. Dev. 2024. https://doi.org/10.1021/acs.oprd.4c00136
Abstract: 2-Methyl benzothiazoles are widely used as key precursors for dyes, photosensitizers, and fluorescent markers. Thus, they are demanded in multigram and even kilogram quantities. We propose a scalable single-step procedure for producing 2-alkyl-substituted benzothiazoles from corresponding bis(2-nitrophenyl)disulfides of commercial, technical-grade quality. The reaction scope looks promising; substrates containing various substituents, including carboxylic and ester groups, were introduced. The reaction conditions were carefully optimized according to reducing agents (diverse sodium salts), solvents, ratio, and reaction time. This led to obtaining target products in up to 350 g quantities in a single run.
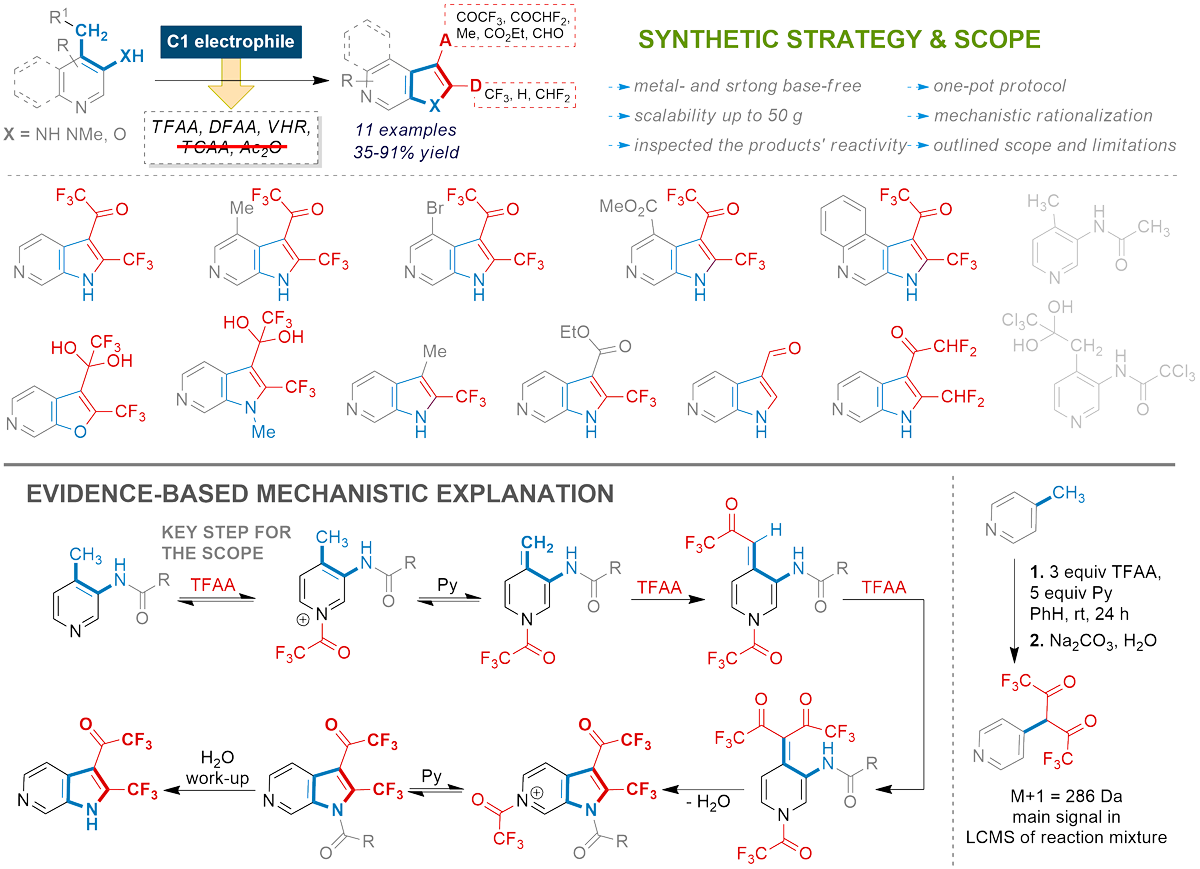
New horizons of 6-azaindoles
Ivonin, S. P.; Voloshchuk, V. V.; Rusanov, E. B.; Suikov, S.; Ryabukhin, S. V.; Volochnyuk, D. M. Synthesis of 6-azaindoles via formal electrophilic [4 + 1]-cyclization of 3-amino-4-methyl pyridines: new frontiers of diversity. Organic Chemistry Frontiers 2024, 11 (7), 2088-2094. https://doi.org/10.1039/D3QO01937C
Abstract: A scalable and efficient synthesis of 2-trifluoromethyl-3-trifluoroacetyl-6-azaindoles from 3-amino-4-methylpyridines under treatment with TFAA is disclosed. The reaction scope and limitations of pyridine and electrophilic components were investigated. Activation of the methyl group in aminomethylpyridines under mildly acidic conditions appeared promising for significantly expanding the scope of principally novel substrates. Examining the pyridine components using trifluoroacetic anhydride (TFAA) as a model electrophile allowed us to divide them into three groups with different impacts on the reaction outcome. Among the electrophilic components, difluoroacetic anhydride (DFAA), trichloroacetic anhydride (TCAA) and Vilsmeier–Haack reagent (VHR) were inspected. Combining the results led to a cyclization mechanism rationalizing our observations.
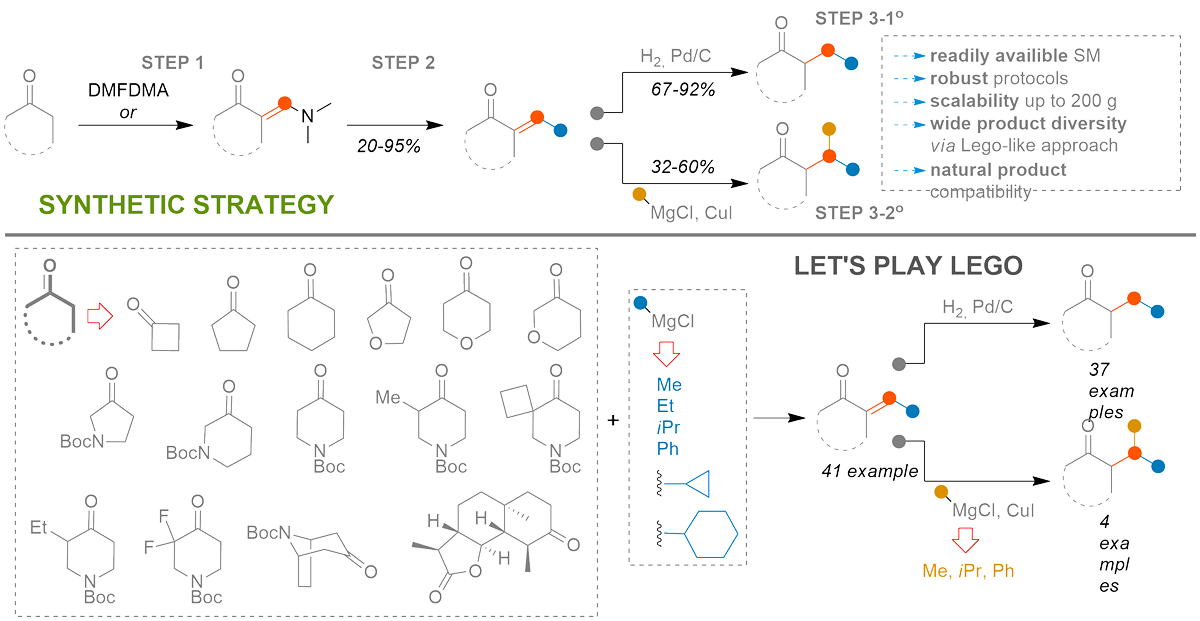
The power of chemical constructor
Frolov, A. I.; Chuchvera, Y. O.; Ostapchuk, E. N.; Druzhenko, T. V.; Volochnyuk, D. M.; Ryabukhin, S. V. Toward a Chemical Constructor: A Lego-Like Approach for Formal α-Alkylation of Cyclic Ketones. J. Org. Chem. 2024, 89 (11), 8208-8219. https://doi.org/10.1021/acs.joc.3c02628
Abstract: A conceptual strategy for a formal α-alkylation of α-methylene ketones was developed. Diverse 1° and 2° alkyl substituents were generated in the α-position of various ketones via the synthesis of enaminone and its subsequent treatment with organomagnesium reagents followed by catalytic hydrogenation (1° alkyl) or interaction with organocopper reagents (2° alkyl). Tolerance toward ester, Boc-protected amine, and α-fluoro-substituents in the starting ketone was demonstrated. The suitability of the method for late-stage natural product modification was shown as well.