- 2-Oxabicyclo[2.1.1]hexanes: synthesis, properties and validation as bioisosteres of ortho- and meta-Benzenes
- Spiro[3.3]heptane as a Saturated Benzene Bioisostere
- Light-enabled scalable synthesis of bicyclo[1.1.1]pentane halides and their functionalizations
- 2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
- 1-Azaspiro[3.3]heptane as a Bioisostere of Piperidine
1. 2-Oxabicyclo[2.1.1]hexanes: synthesis, properties and validation as bioisosteres of ortho- and meta-Benzenes
V. V. Levterov, Y. Panasiuk, O. Shablykin, O. Stashkevych, K. Sahun, A. Rassokhin, I. Sadkova, D. Lesyk, A. Anisiforova, Y. Holota, P. Borysko, I. Bodenchuk, N. M. Voloshchuk, P. K. Mykhailiuk. Angew. Chem. Int. Ed. 2024, 63, e202319831 (https://doi.org/10.1002/anie.202319831).
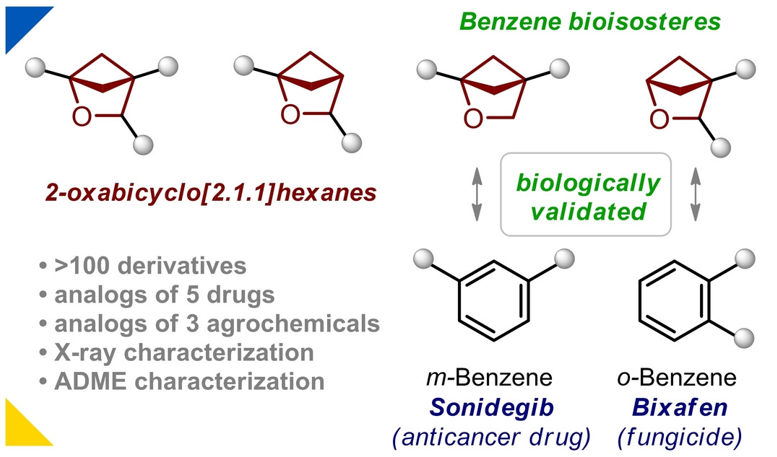
Abstract
We have developed a general and practical approach towards 2-oxabicyclo[2.1.1]hexanes with two and three exit vectors via an iodocyclization reaction. The obtained compounds have been easily converted into the corresponding building blocks for use in medicinal chemistry. 2-Oxabicyclo[2.1.1]hexanes have been incorporated into the structure of five drugs and three agrochemicals, and validated biologically as bioisosteres of ortho- and meta-benzenes.
2. Spiro[3.3]heptane as a Saturated Benzene Bioisostere
K. Prysiazhniuk, O. P. Datsenko, O. Polishchuk, S. Shulha, O. Shablykin, Y. Nikandrova, K. Horbatok, I. Bodenchuk, P. Borysko, D. Shepilov, I. Pishel, V. Kubyshkin, P. K. Mykhailiuk. Angew. Chem. Int. Ed. 2024, 63, e202316557 (https://doi.org/10.1002/anie.202316557).
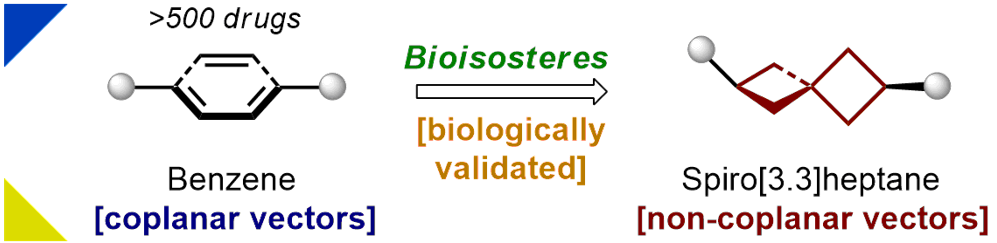
Abstract
The spiro[3.3]heptane core, with the non-coplanar exit vectors, was shown to be a saturated benzene bioisostere. This scaffold was incorporated into the anticancer drug sonidegib (instead of the meta-benzene), the anticancer drug vorinostat (instead of the phenyl ring), and the anesthetic drug benzocaine (instead of the para-benzene). The patent-free saturated analogs obtained showed a high potency in the corresponding biological assays.
3. Light-enabled scalable synthesis of bicyclo[1.1.1]pentane halides and their functionalizations
V. Ripenko, V. Sham, V. Levchenko, S. Holovchuk, D. Vysochyn, I. Klymov, D. Kyslyi, S. Veselovych, S. Zhersh, Y. Dmytriv, A. Tolmachev, I. Sadkova, I. Pishel, K. Horbatok, V. Kosach, Y. Nikandrova, P. K. Mykhailiuk. Nat. Synth (2024). (https://doi.org/10.1038/s44160-024-00637-y)
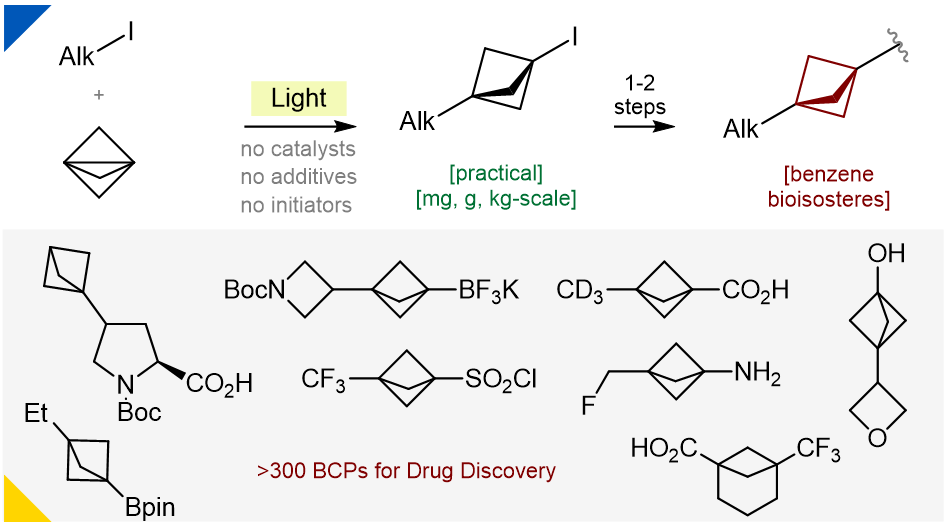
Abstract
In 2012, bicyclo[1.1.1]pentanes were demonstrated to be bioisosteres of the benzene ring. Here, we report a general scalable reaction between alkyl iodides and propellane that provides bicyclo[1.1.1]pentane iodides in milligram, gram and even kilogram quantities. The reaction is performed in flow and requires just light; no catalysts, initiators or additives are needed. The reaction is clean enough that, in many cases, evaporation of the reaction mixture provides products in around 90% purity that can be directly used in further transformations without any purification. Combined with the subsequent functionalization, >300 bicyclo[1.1.1]pentanes for medicinal chemistry have been prepared. So far, this is the most general and scalable approach towards functionalized bicyclo[1.1.1]pentanes.
4. 2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
V. V. Levterov, Y. Panasiuk, K. Sahun, O. Stashkevych, V. Badlo, O. Shablykin, I. Sadkova, L. Bortnichuk, O. Klymenko-Ulianov, Y. Holota, L. Lachmann, P. Borysko, K. Horbatok, I. Bodenchuk, Y. Bas, D. Dudenko, P. K. Mykhailiuk. Nat. Commun. 2023, 14, 5608. (https://doi.org/10.1038/s41467-023-41298-3)
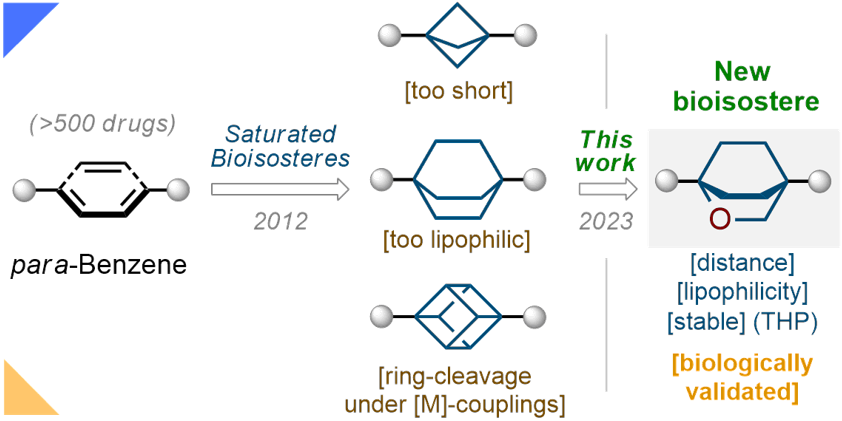
Abstract
The phenyl ring is a basic structural element in chemistry. Here, we show the design, synthesis, and validation of its new saturated bioisostere with improved physicochemical properties − 2-oxabicyclo[2.2.2]octane. The design of the structure is based on the analysis of the advantages and disadvantages of the previously used bioisosteres: bicyclo[1.1.1]pentane, bicyclo[2.2.2]octane, and cubane. The key synthesis step is the iodocyclization of cyclohexane-containing alkenyl alcohols with molecular iodine in acetonitrile. 2-Oxabicyclo[2.2.2]octane core is incorporated into the structure of Imatinib and Vorinostat (SAHA) drugs instead of the phenyl ring. In Imatinib, such replacement leads to improvement of physicochemical properties: increased water solubility, enhanced metabolic stability, and reduced lipophilicity. In Vorinostat, such replacement results in a new bioactive analog of the drug. This study enhances the repertoire of available saturated bioisosteres of (hetero)aromatic rings for the use in drug discovery projects.
5. 1-Azaspiro[3.3]heptane as a Bioisostere of Piperidine
A. A. Kirichok, H. Tkachuk, Y. Kozyriev, O. Shablykin, O. Datsenko, D. Granat, T. Yegorova, Y. P. Bas, V. Semirenko, I. Pishel, V. Kubyshkin, D. Lesyk, O. Klymenko-Ulianov, P. K. Mykhailiuk. Angew. Chem. Int. Ed. 2023, 62, e202311583 (https://doi.org/10.1002/anie.202311583)
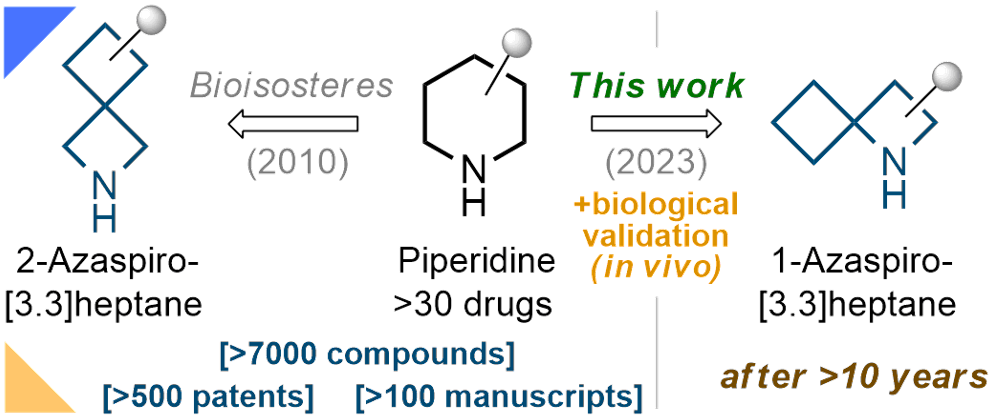
Absract
1-Azaspiro[3.3]heptanes were synthesized, characterized, and validated biologically as bioisosteres of piperidine. The key synthesis step was thermal [2+2] cycloaddition between endocyclic alkenes and the Graf isocyanate, ClO2S−NCO, to give spirocyclic β-lactams. Reduction of the β-lactam ring with alane produced 1-azaspiro[3.3]heptanes. Incorporation of this core into the anesthetic drug bupivacaine instead of the piperidine fragment resulted in a new patent-free analogue with high activity.
