Tailored towards your needs!
Amines are among the most common functional groups found in various medicinal products and play a significant role in numerous biological systems. They are crucial in the development and effectiveness of medicines, ensuring their activity, stability, and bioavailability. Therefore, the knowledge of the drug's pKa, a key physicochemical property, should be considered one of the primary steps in the drug discovery process, making it highly relevant in medicinal chemistry.
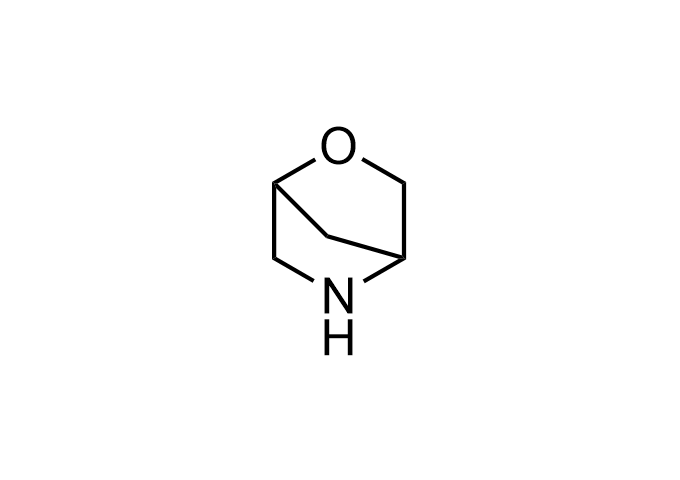
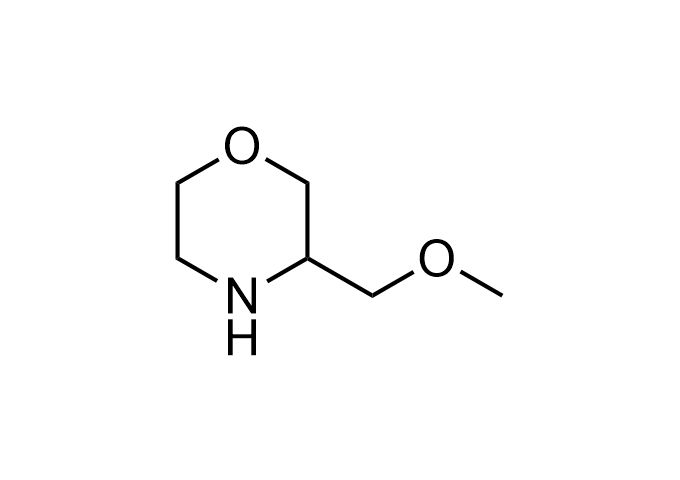
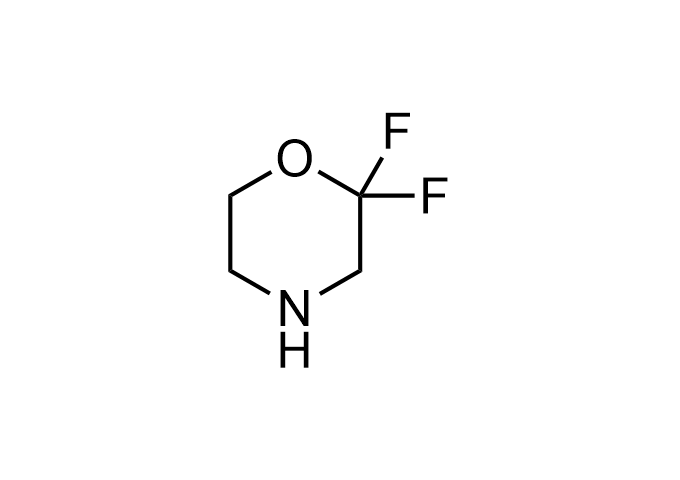
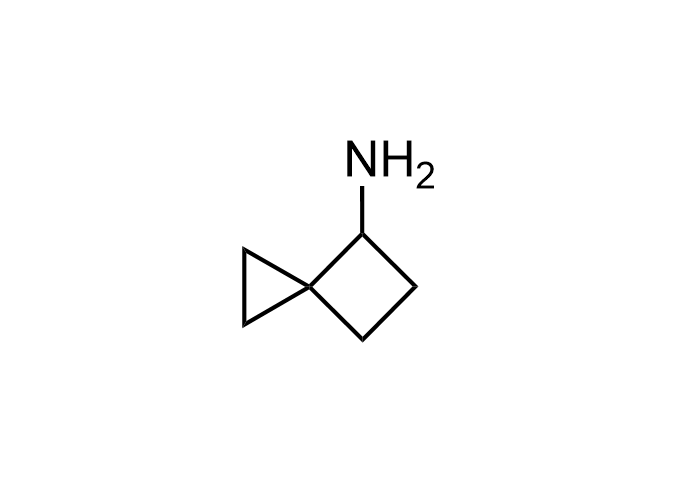
Enamine Basicity-Tuning Kits - are amines that have been specifically designed or modified to adjust their pKa values.
Amines with tuned pKa values are important in pharmaceutical chemistry and in the design of medicinal products for several reasons:
- Solubility control: The pKa values of amines can affect their solubility in water and other solvents. This is important to ensure the effective distribution and transportation of pharmaceutical preparations in biological environments;
- Bioavailability: Tuned pKa can help ensure faster absorption of medicinal preparations into the body, as they can more efficiently penetrate biological barriers and membranes;
- Activity control: Tuning the pKa can affect the activity of medicinal substances, providing better compatibility with biological targets or reducing the potential for side effects. This allows for a more precise and balanced influence on biological processes;
- Structure optimization: Adjusting the pKa allows chemists to optimize the structure of drug molecules to achieve desired physical and physicochemical properties.
Thus, basicity influences many characteristics of pharmaceuticals, including ADME.
Enamine offers:
Collections of various amines tuned across a wide pKa range, specifically from <2.5 to>12 (mostly 5-11). These Enamine Basicity-Tuning Kits were developed by our highly qualified specialists to evenly distribute them across the pKa scale as much as possible. Our clients can find amines with almost any pKa value to address specific challenges in drug discovery.
Our catalog includes 18 sets -3 sets per each chemical compound class - azetidine, pyrrolidine, piperidine, morpholine, cyclobutyl and cyclopropyl-modified amino moieties. The first set – Basic set - consists of 7 amines that are most widely and commonly used in medicinal chemistry. The second one – Advanced set- includes 7 amines that are the most modern, rare, and unique compounds that will expand drug discovery processes. The third set – Pro set - is the extended set, which consists of 15 Enamine Basicity-Tuning Kits that can satisfy any needs in MedChem research.
All compounds from these collections undergo extensive quality control by NMR and LC-MS, and purity is guaranteed to be over 95% and are available in our stock. All pKa values have been measured experimentally by our analytical department through potentiometric titration.
Our specialists continue to work on further expansion and diversification of these sets of pKa-tuned amines, adding new classes of amino compounds, to advance and accelerate the efforts in drug discovery.
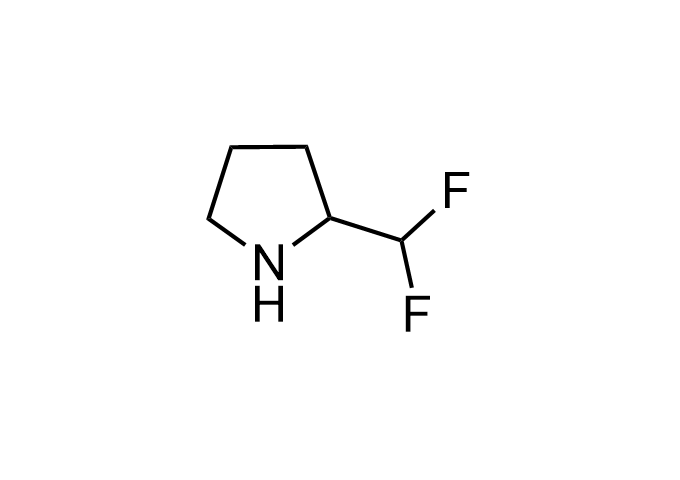
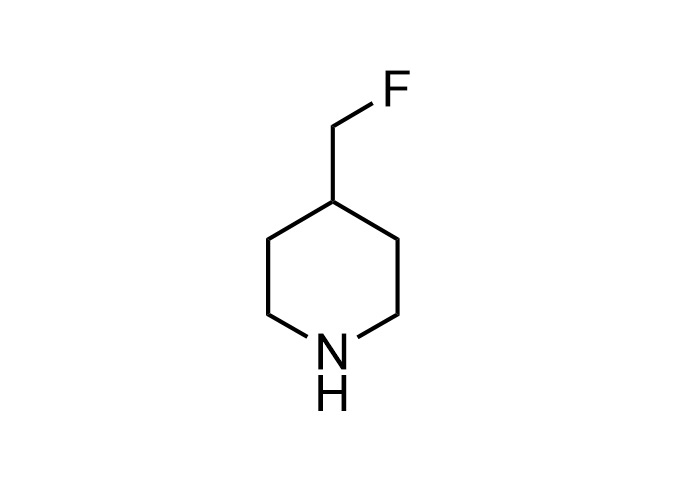
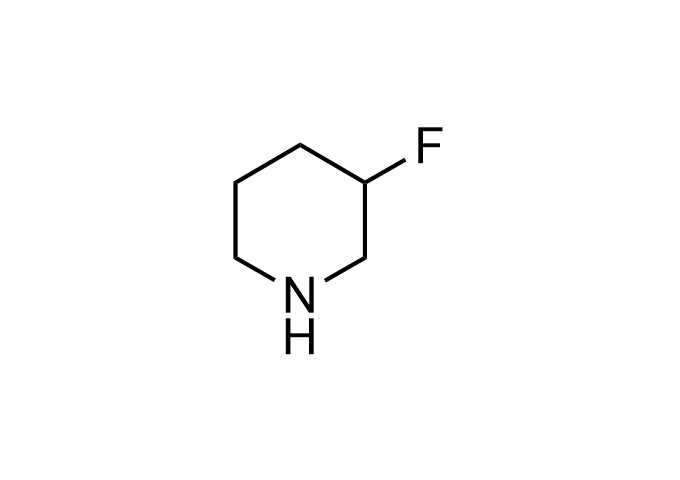
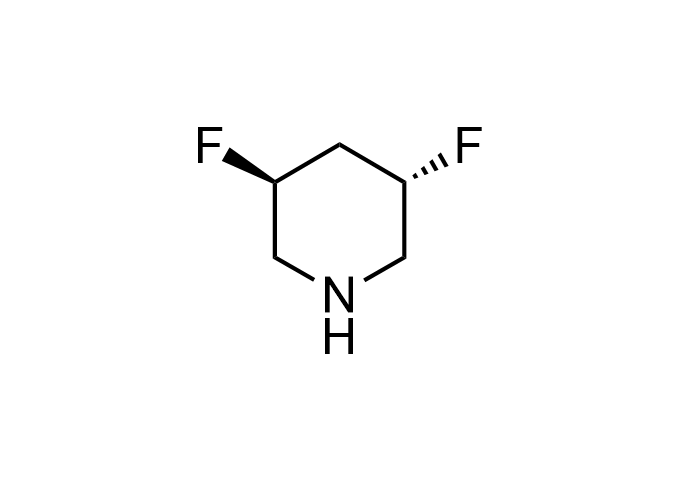
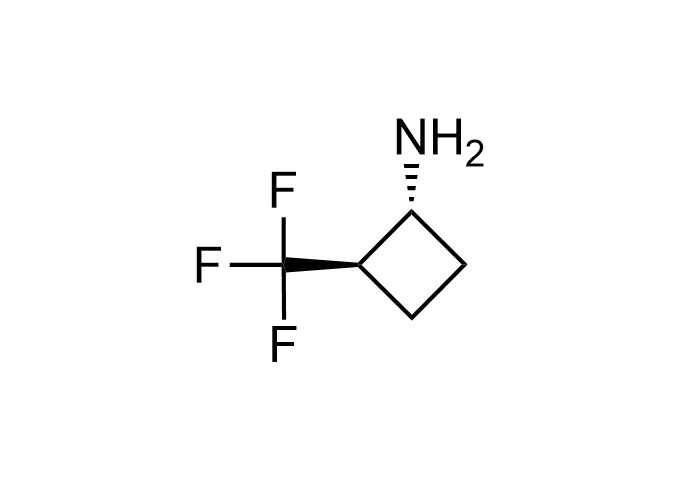
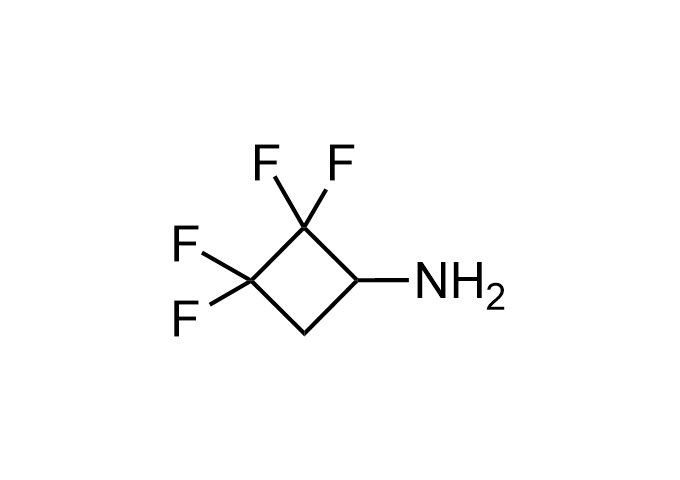
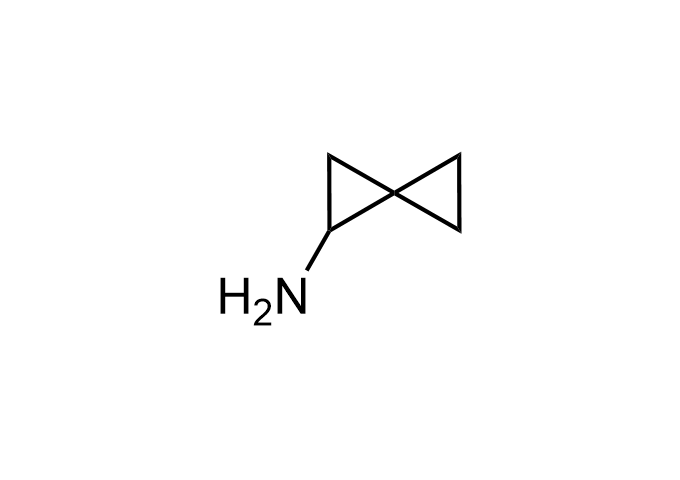
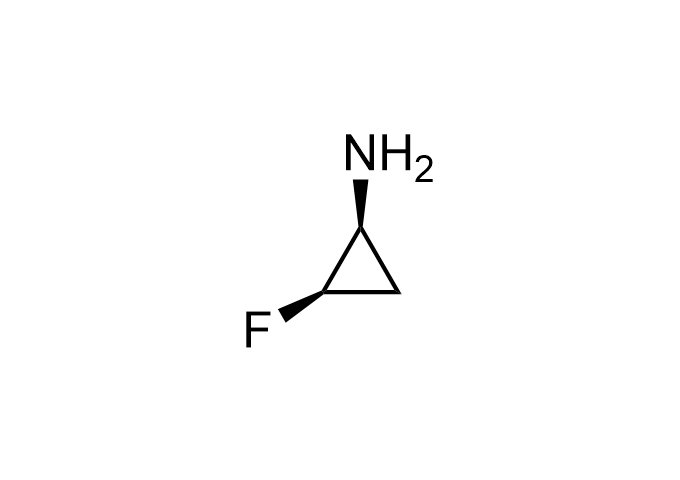
Examples of Enamine Basicity-Tuning Kits with the values of their pKa:
Frequently Asked Questions
Selected publications on the use of Enamine Basicity-Tuning Kits:
- The pKa Distribution of Drugs: Application to Drug Discovery.
Manallack D. T. Perspectives in Medicinal Chemistry 2007, 1. DOI: 10.1177/1177391X0700100003 - The significance of acid/base properties in drug discovery.
Manallack D. T., Prankerd R. J., Yuriev E., Oprea T. I., Chalmers D. K. Chem. Soc. Rev. 2013, 42, 2, 485–496. DOI: 10.1039/C2CS35348B - Best of both worlds: An expansion of the state of the art pKa model with data from three industrial partners.
Fraczkiewicz R., Quoc Nguyen H., Wu N., Kausch-Busies N., Grimbs S., Sommer K., ter Laak A., Günther J., Wagner B., Reutlinger M. Molecular Informatics 2024, 43:e202400088. DOI: 10.1002/minf.202400088?af=R
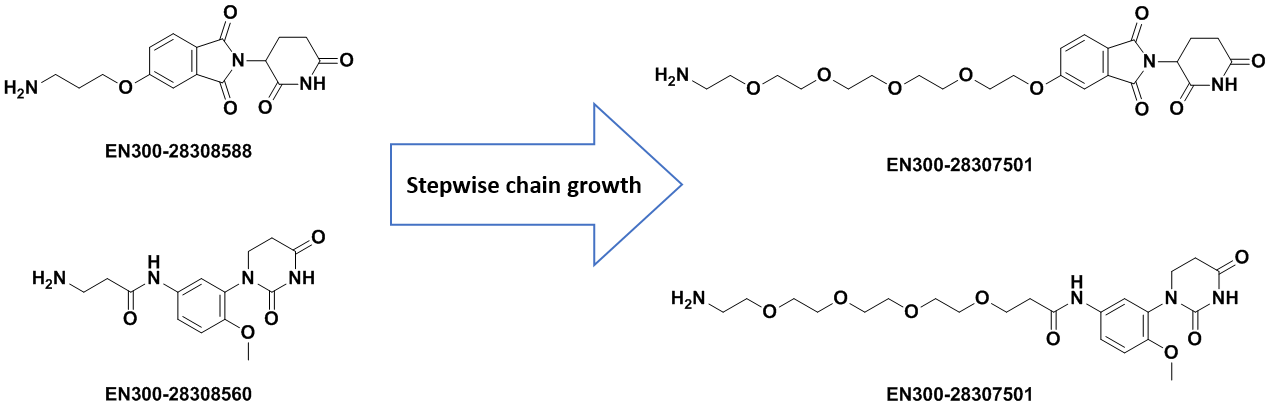
We at Enamine are constantly working on synthesizing new molecules and elaborating new synthetic approaches to facilitate access to the most demanded chemistry and intermediates for the Drug Discovery Community. We carefully designed and synthesized a series of E3 binders with attached linkers of different lengths and natures to support Degrader Research and Development. Our primary focus has been the derivatization of Cereblon (CRBN) binders since they are most frequently used in constructing PROTAC molecules.
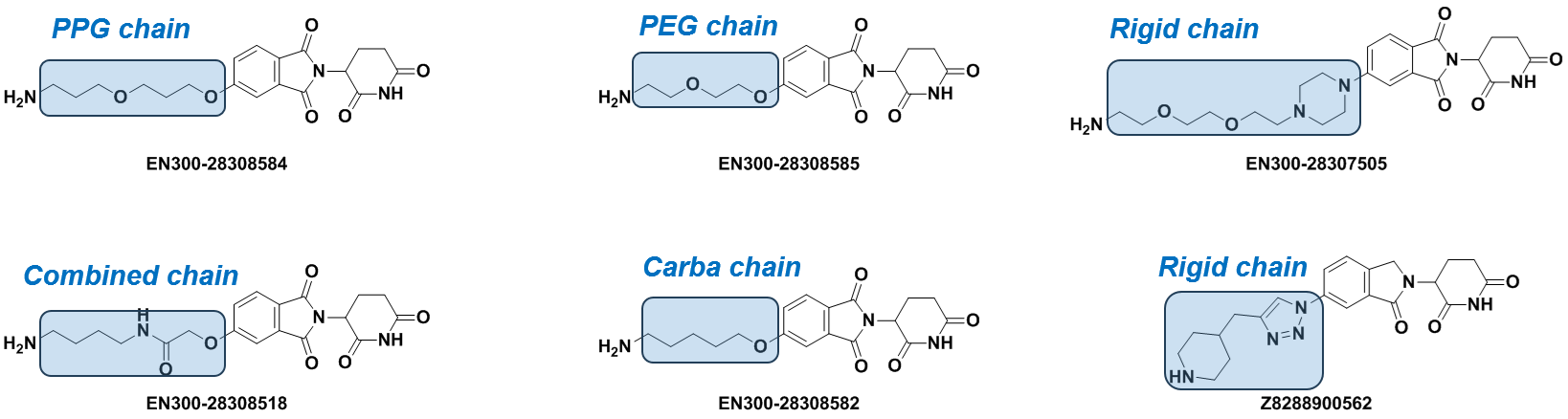
The Kits represented here include commonly used CRBN ligands functionalized at C4 and C5 positions to avoid interference with binding affinity. The attached ligands are of variable lengths from 2 to up to 18 heavy atoms in the chain or as a part of a more rigid construction.
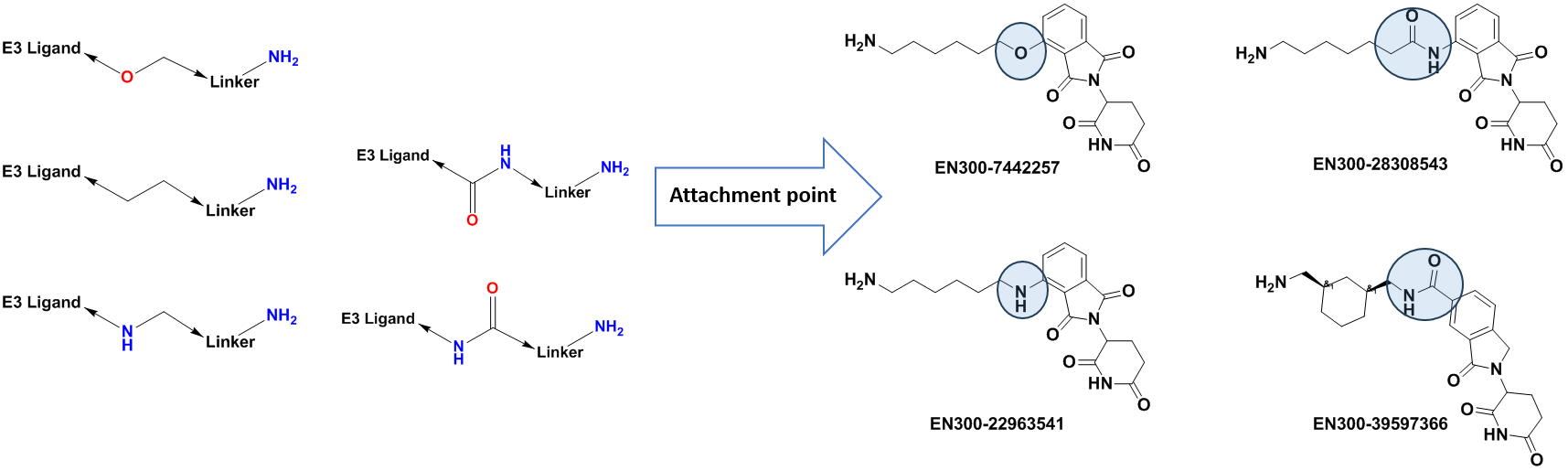
Apart from the standard PEG and Carba-linkers, we offer intermediates with rigid/cyclic linkers that have been successfully utilized in the design of bifunctional molecules. Linkers terminal functional groups can be easily modified through the most straightforward reactions – amide coupling or click-chemistry.
Product catalog
Product name
CRBN Amine Kit-1
Amine-1-96
Description
Thalidomide-based ligands derivatized with most comprehensive and diverse linkers of variable length from 2 to 18 heavy atoms in a chain with terminal amine functional group
Product name
CRBN Amine Kit-2
Amine-2-35
Description
Uracyl-based Cereblon ligands with linear PEG- and Carba-linkers with terminal amine group able for modification
Product name
CRBN Azide Kit-1
Azide-1-28
Description
Thalidomide-based ligands with linear PEG- and Carba-linkers with terminal azide function for Click chemistry
Key features
- Easy access, ready for delivery in plates as 50 mM and 20 mM solutions in DMSO
-
Variable in length from 2 up to 18 heavy atoms
-
Well-balanced composition including PEG-, Carba- and Rigid-linkers
-
Variation of linker conjugation point
Ready-to-use sets to support your discoveries
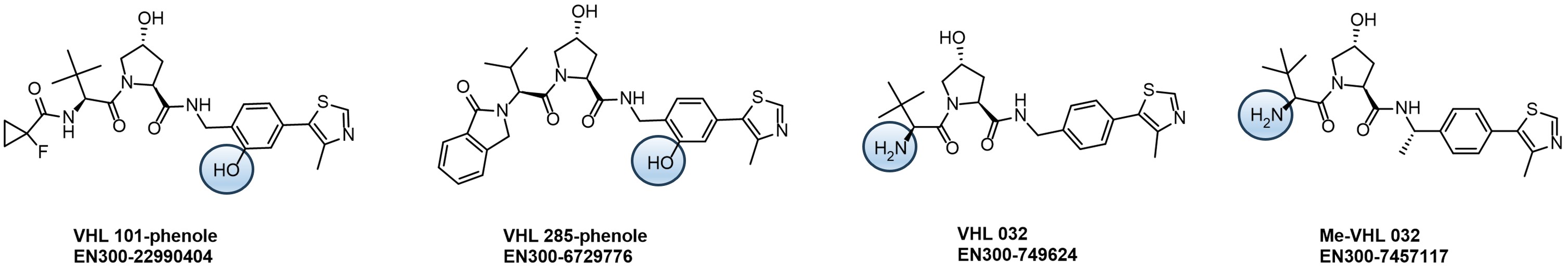
We at Enamine are constantly working on creating novel molecules and innovative synthetic pathways for the Drug Discovery Community. We carefully designed and synthesized a series of E3 binders with attached linkers of different lengths and natures to support Degrader Research and Development. One of our recent focuses is the derivatization of the von Hippel-Lindau (VHL) Cullin RING E3 ligase binders.
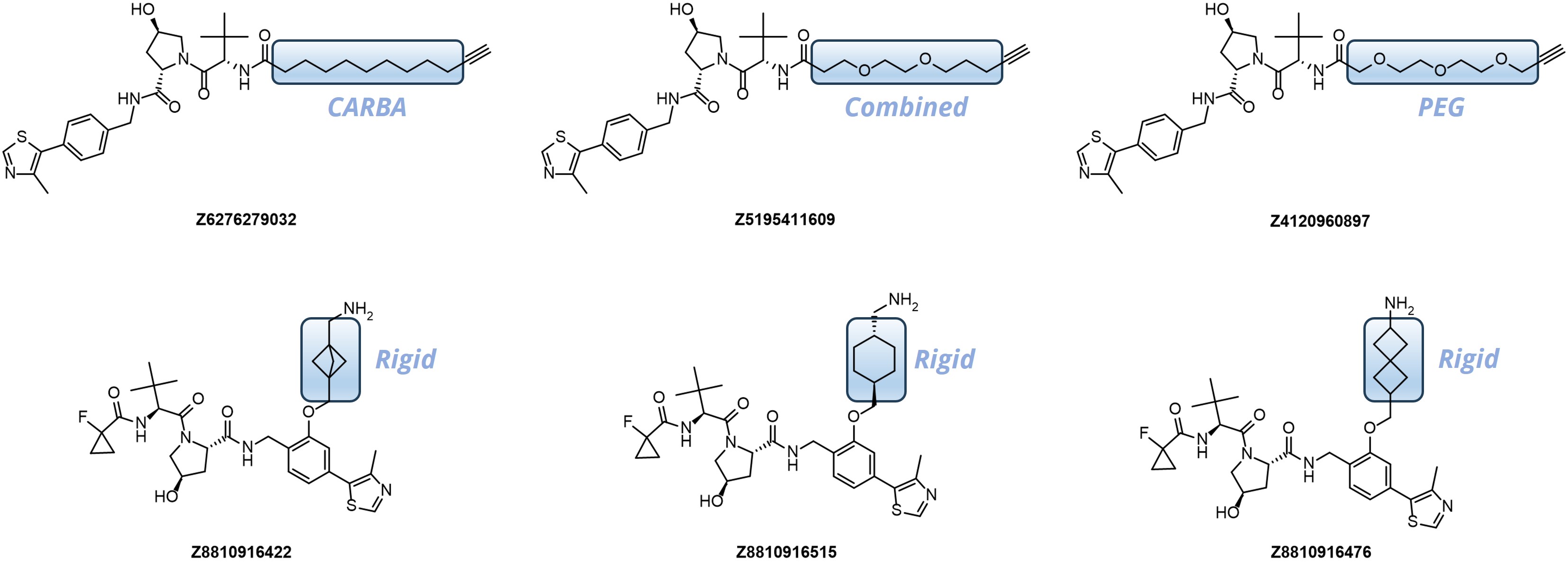
Our kits provide a robust selection of pre-synthesized VHL ligands (VHL 032, Me-VHL 032, VHL 101-phenol, and VHL 285-phenol) conjugated to linkers with varying lengths and compositions. This comprehensive offer goes beyond standard PEG- and Carba-linkers, including rigid/cyclic linkers proven effective in bifunctional molecule design.
Linker’s terminal functional groups can be easily modified through the most straightforward reactions – amide coupling or click-chemistry.
Product catalog
Product name
VHL Amine Kit-1
Amine-1-227
Description
VHL 032 and Me-VHL 032 derivatized with most comprehensive and diverse linkers of variable length from 2 to 18 heavy atoms in a chain with terminal amine functional group
Key features
- Easy access, ready for delivery in plates as 20 mM solutions in DMSO
- Variation VHL ligand
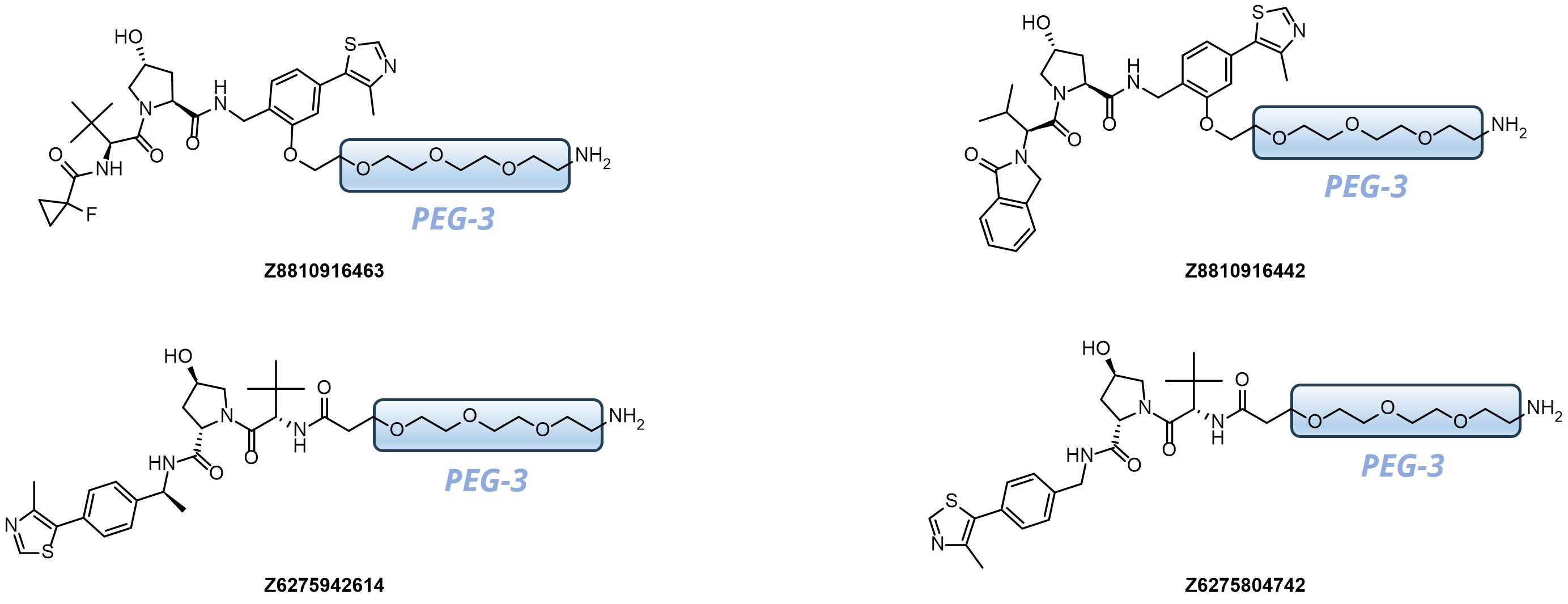
- Variable in length from 4 up to 18 heavy atoms
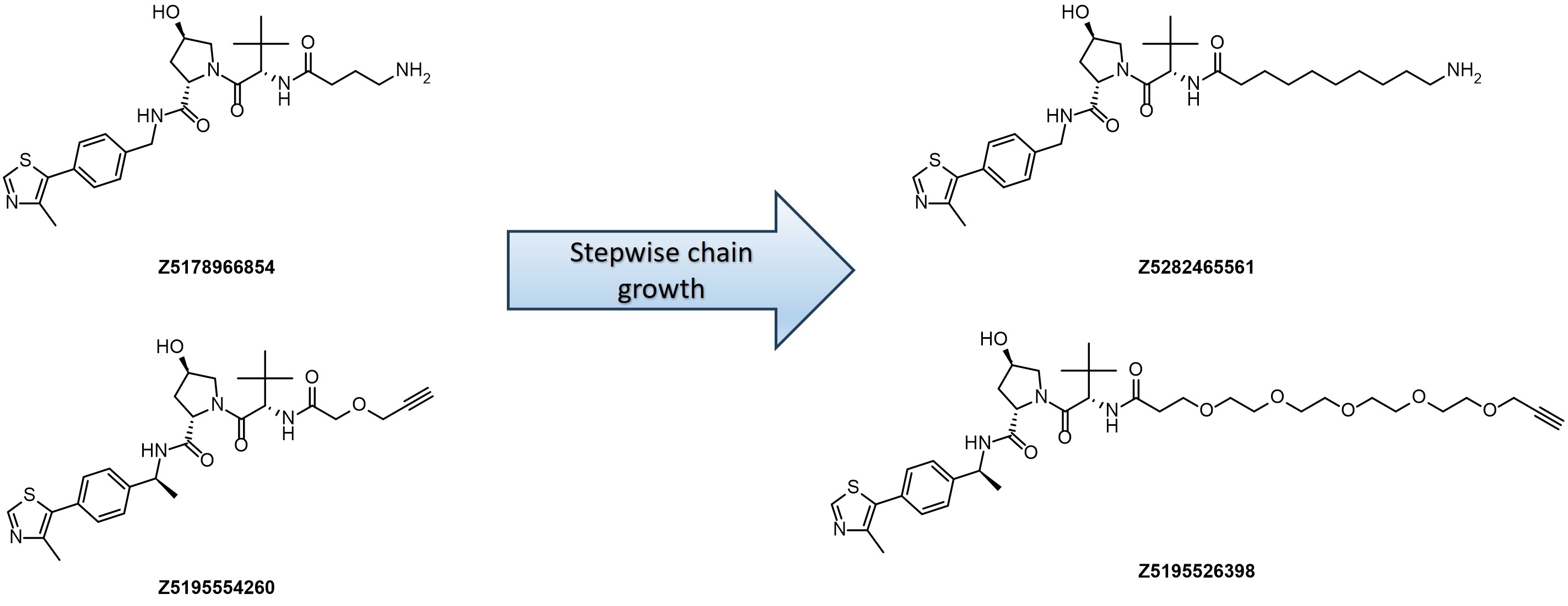
- Well-balanced composition including PEG-, Carba- and Rigid-linkers
Screening collections play a pivotal role in early-stage drug discovery, providing a flexible platform compatible with many advanced methods. Our collaboration with UORSY has led to the creation of a joint collection of screening compounds named 'Legacy', reflecting the rich scientific heritage of Ukrainian suppliers. This collection has resulted from our 15 years of efforts in supporting drug discovery through high-throughput screening and comprises over 1.7 million in-stock screening compounds.
Download SD file
We provide compounds synthesized from in-house synthesized building blocks using meticulously developed and optimized reactions. More than 50% of our compounds have drug-like physicochemical properties, and over 40% - lead-like profiles.
Key features:
- 11.2% shaped molecules (9.3% disc & 1.9% sphere)
- 4.9% high QED (QED=0.9)
- 3.7% PAINS
- 2.7% fragment-like compounds
- 0.8% natural-like compounds
- 100% affordable prices
Availability, packaging, and quality control
The compounds are available in milligram amounts, typically 1-20 mg, sufficient for a fast re-supply of the hits. The samples are mainly stored as dry powders and can be supplied either neat or as DMSO solutions in virtually any applicable format. All Enamine compounds undergo rigorous quality control with LCMS and/or 1H NMR to meet the standard of at least 90% purity.
Selecting the right compounds for your project
Our experienced team of Medicinal & Computational chemistry team is ready to assist you choose a hit-finding strategy tailored to your specific needs.
Discover the Legacy Screening Collection on , where you can explore compounds using text, substructure, and similarity enaminestore.com queries.
9 085 compounds in stock
1 360 pre-plated compounds
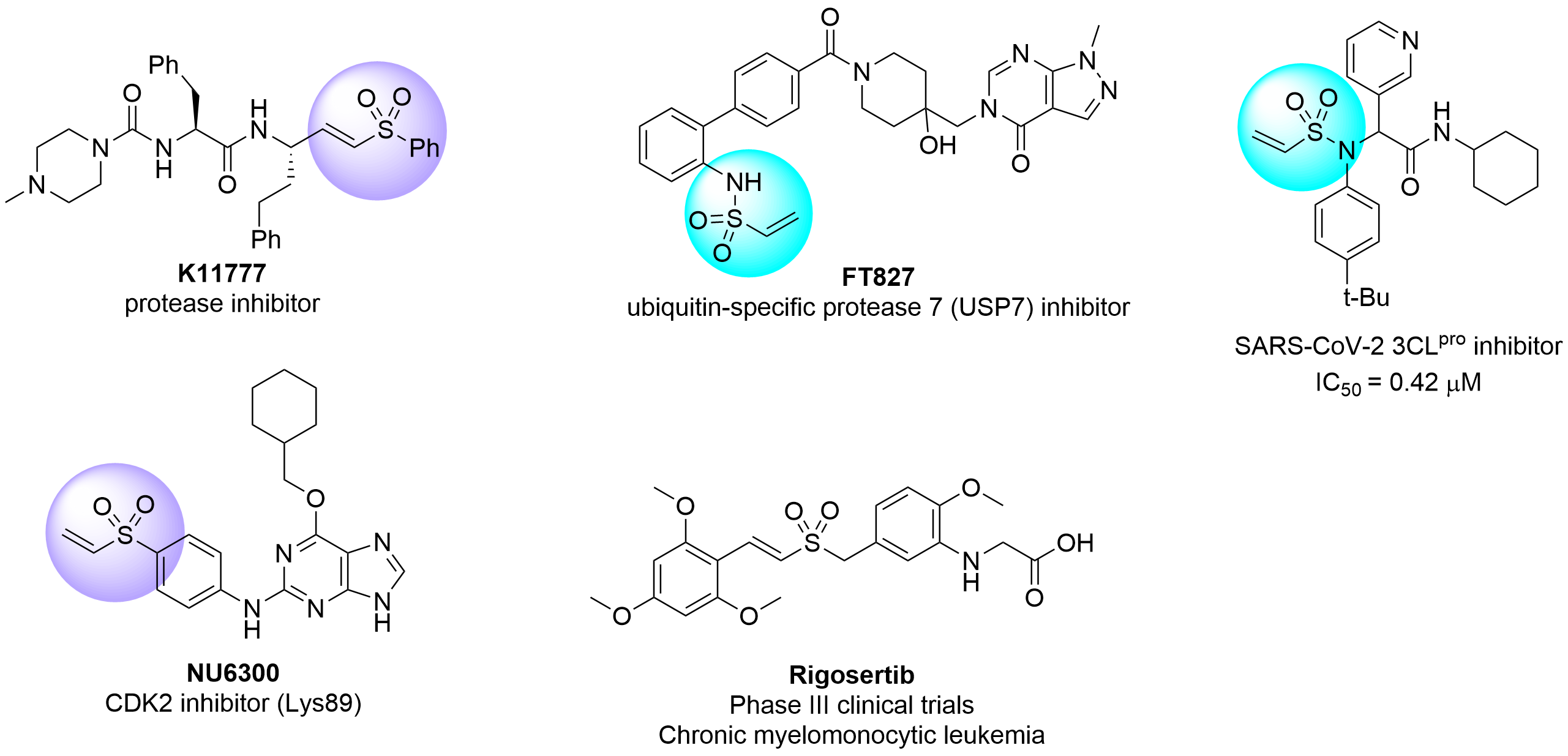
Vinyl sulfones and sulfonamides are among the most common and efficient Michael acceptors and are good alternatives to acrylamides. This class of covalent binders attracted much attention with several representatives being in different stages of clinical trials. The recent investigation described by Adam Gilbert and co-workers using N-acetyl lysine and glutathione showed that vinyl sulfonyl derivatives in general are more reactive than acrylamides and sulfones are more active over sulfonamides. Last but not least vinyl sulfone and vinyl sulfonamide warheads displayed some selectivity toward Lys over cysteine residue if compared to popular acrylamide electrophiles.
Several recent examples of successful application of vinyl sulfones in drug discovery include irreversible protease inhibitor K11777 targeting noncatalytic Cys25, the first irreversible ATP-competitive inhibitor of CDK2 NU6300 targeting Lys89, FT827 potent covalent inhibitor of USP7 and promising SARS-CoV-2 Main protease inhibitor which covalently binds to Cys145.
Download SD files
9 085 compounds for cherry-picking
Plated libraries in stock:
Version: 9 January 2024
320 pre-plated compounds
sublibrary of LYS-1600
Examples of vinyl sulfones and sulfonamides in pre-plated sets