Tetrahedron Lett. , 2013, 54 (14), 1897-1898
DOI: 10.1016/j.tetlet.2013.01.132
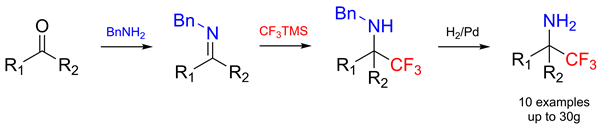
A small library of structurally diverse primary amines bearing a geminal CF3 group was synthesized on a preparative scale. The synthesis starts with an aldehyde or ketone that reacts with benzylamine yielding the corresponding imine. The latter is then trifluoromethylated with Me3SiCF3 under acidic conditions to give a benzylalkylamine. In the last step the Pd-mediated hydrogenation of the benzylalkylamines furnishes the title compounds. All synthetic steps are high-yielding; neither the isolation of the intermediates nor the chromatographic purification of the products is necessary.
Radchenko D. S.; Michurin O. M.; Chernykh A. V.; Lukin O.; Mykhailiuk P. K.
Tetrahedron Lett. 2013, 54 (14), 1897-1898
DOI: 10.1016/j.tetlet.2013.01.132
