Synthesis , 2008, 06, 948-956
DOI: 10.1055/s-2008-1032197
Khodakovskiy P. V.; Volochnyuk D. M.; Panov D. M.; Pervak I. I.; Zarudnitskii E. V.; Shishkin O. V.; Yurchenko A. A.; Shivanyuk A.; Tolmachev A. A.
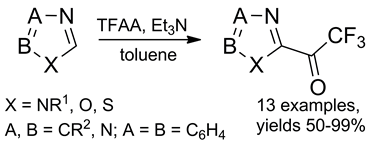
A facile method of trifluoroacylation of imidazoles, 1,3-thiazoles, and 1,3-oxazoles with trifluoroacetic anhydride resulted in a set of heterocyclic trifluoromethyl-containing ketones. Unlike common ketones, these compounds form stable hydrates and enter into noncatalytic ene reactions with terminal olefins affording the corresponding allyl alcohols.
Khodakovskiy P. V.; Volochnyuk D. M.; Panov D. M.; Pervak I. I.; Zarudnitskii E. V.; Shishkin O. V.; Yurchenko A. A.; Shivanyuk A.; Tolmachev A. A.
Synthesis 2008, 06, 948-956
DOI: 10.1055/s-2008-1032197
