Synthesis 2020, 52 (13), 1915-1926
DOI: 10.1055/s-0039-1707987
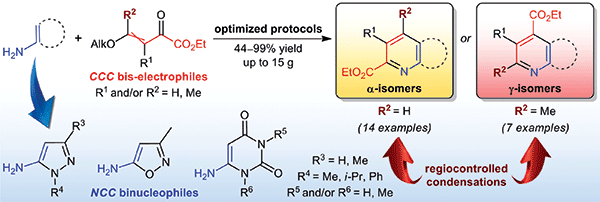
An efficient approach to the preparation of pyridine carboxylates fused with 5- or 6-membered heteroaromatic rings is described. The method relied on the Combes-type condensation of the low-molecular-weight β-alkoxyvinyl glyoxylates as CCC bis-electrophiles and with heteroaromatic amines as NCC binucleophiles. In most experiments, β-alkoxyvinyl glyoxylates without additional substituent at the β position led to the corresponding α-pyridine carboxylates (67–87% yield). In the case of β-methyl-substituted derivative, γ-pyridine carboxylates were obtained in 84–99% yield. It was found that regioselectivity of the condensation could be efficiently tuned by changing conditions, such as solvents and acidic additives (HOAc, DMSO or HCl–1,4-dioxane).