Tetrahedron Lett. 2020, 61 (12)
DOI: 10.1016/j.tetlet.2020.151645
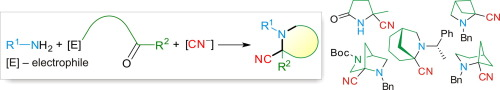
Approaches to α-cyanopyrrolidines, -piperidines, and -azepanes, as well as their bi- and polycyclic analogues are surveyed, which are based on Strecker reaction – intramolecular nucleophilic cyclization. The reactions are categorized according to the nature of the internal electrophile participating in the cyclization step, i.e. carboxylic acid or its derivative, carbonyl compound, or alkylating agent. Special attention is paid to one-pot tandem Strecker reaction – SN2-type nucleophilic cyclization (STRINC), or “cyanide-induced dynamic intramolecular cyclization”, which is an efficient and convenient approach to various mono- and bicyclic α-amino nitriles and α-amino acids.