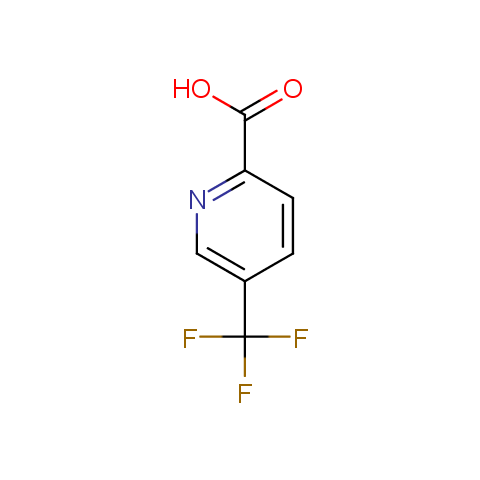
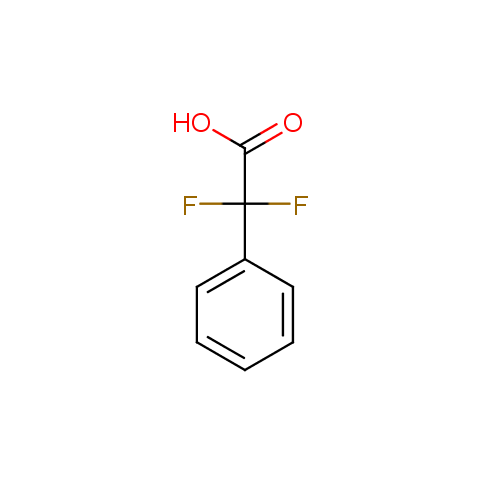
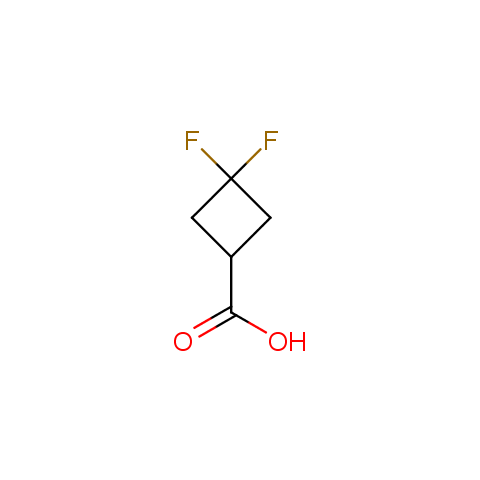
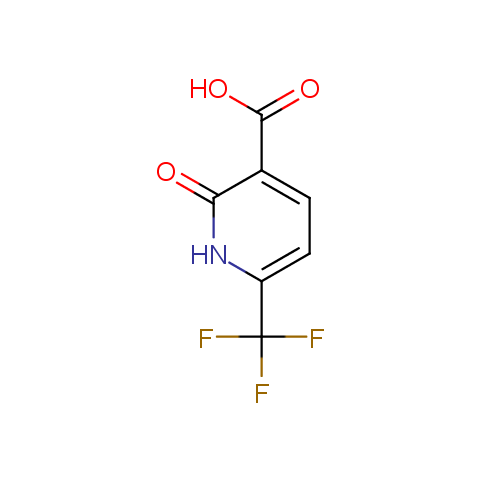
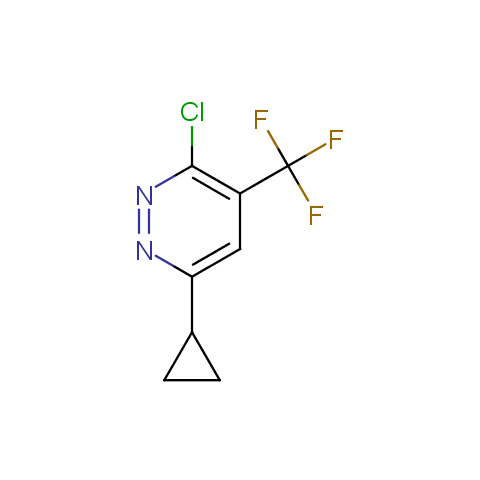
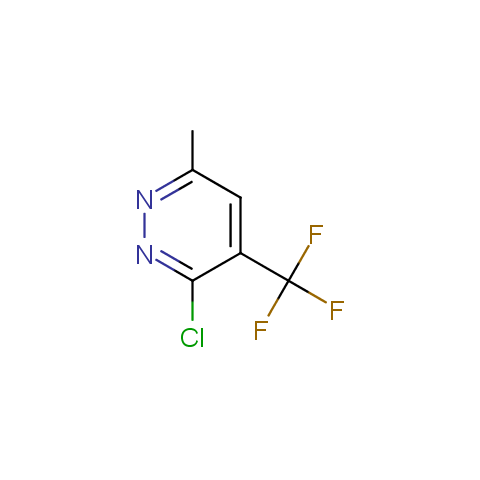
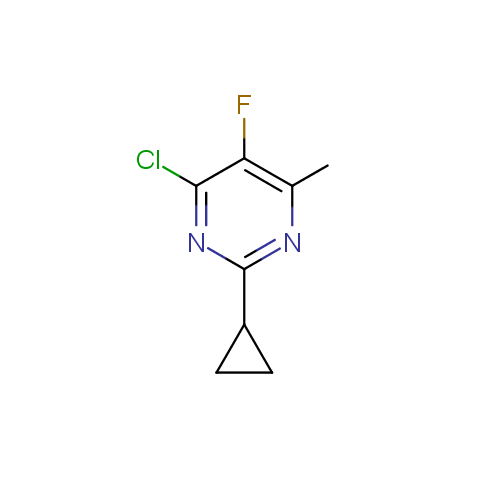
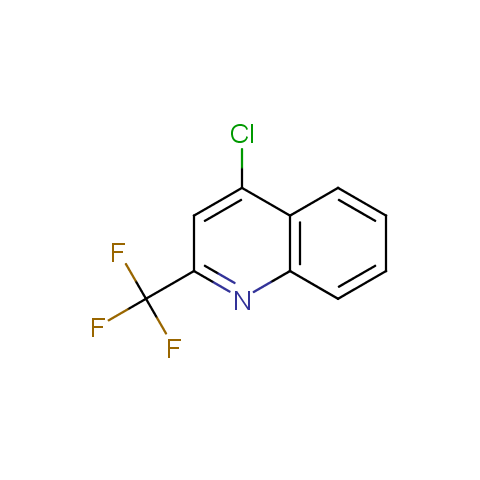
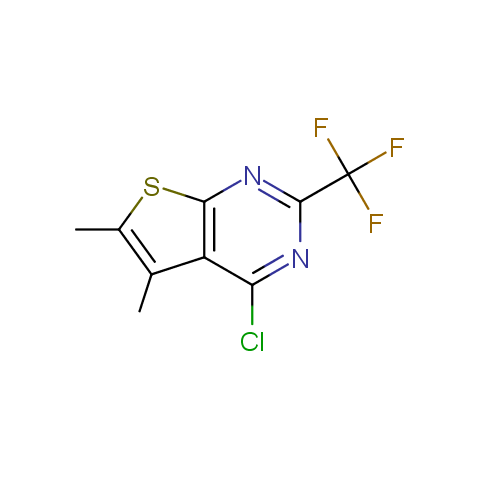
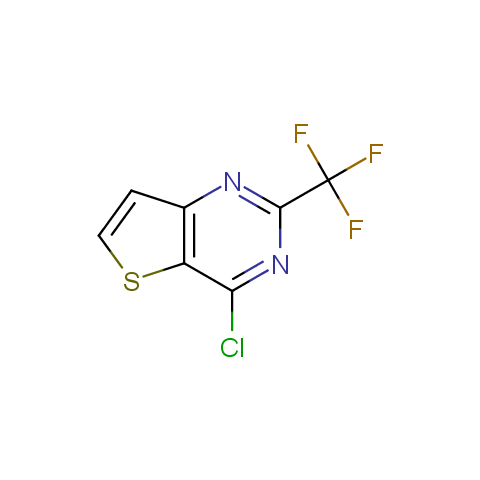
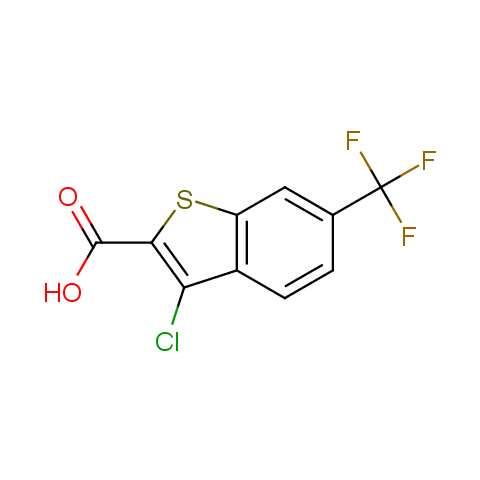
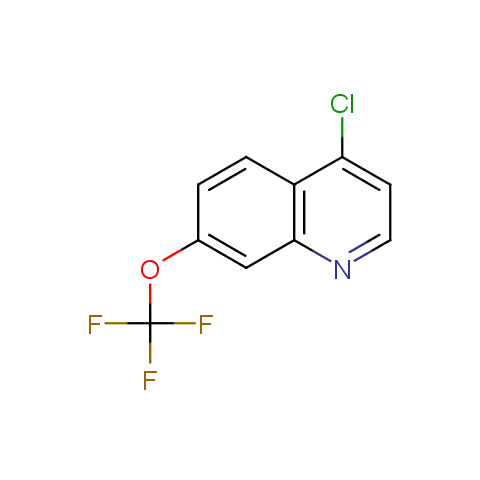
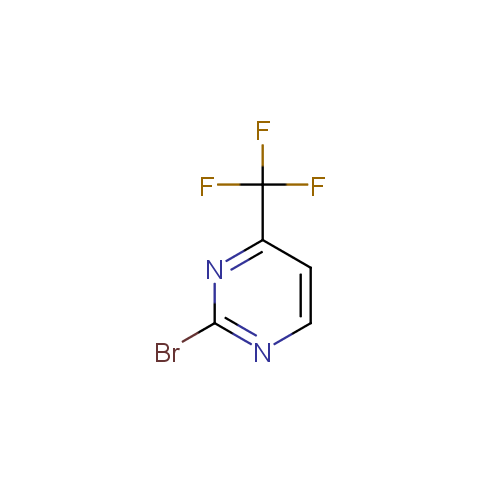
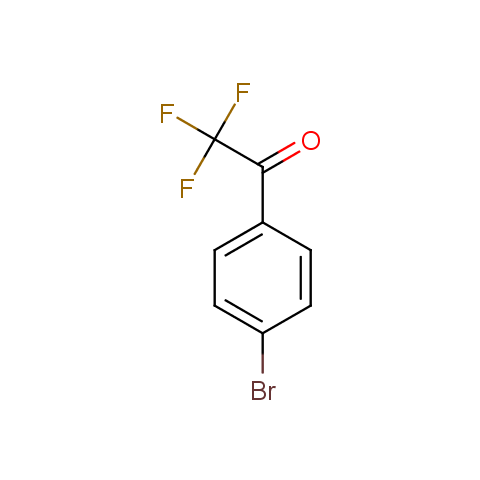
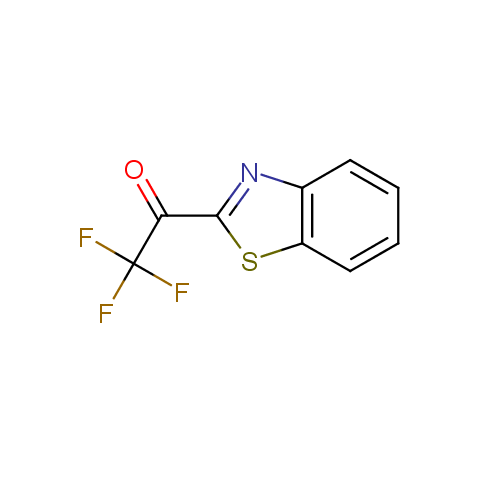
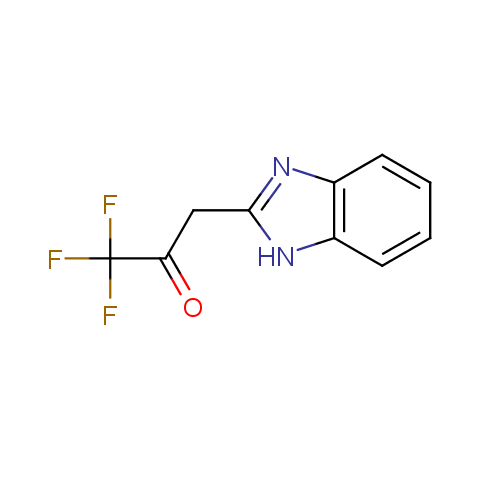
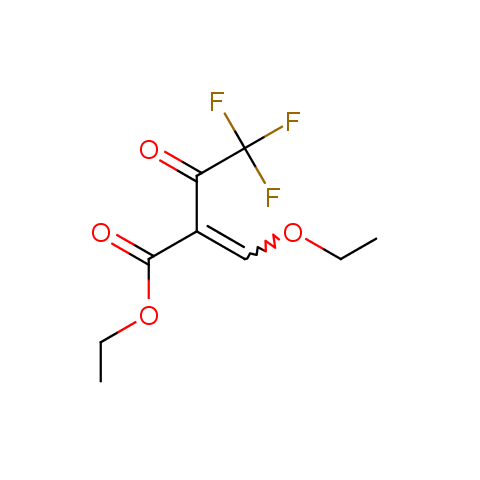
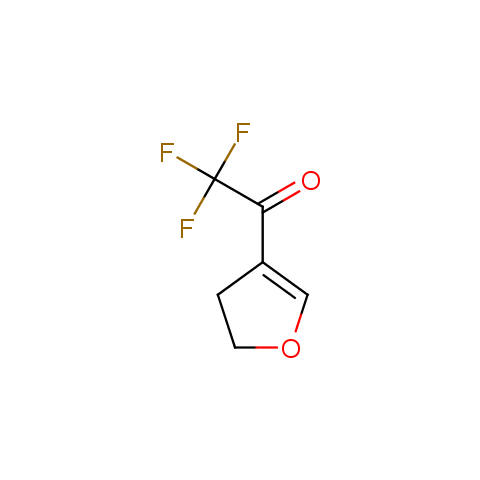
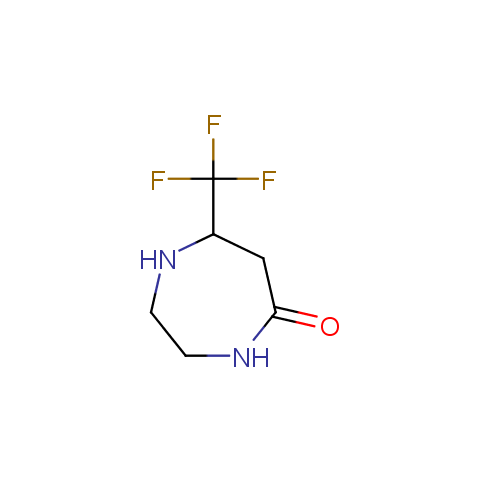
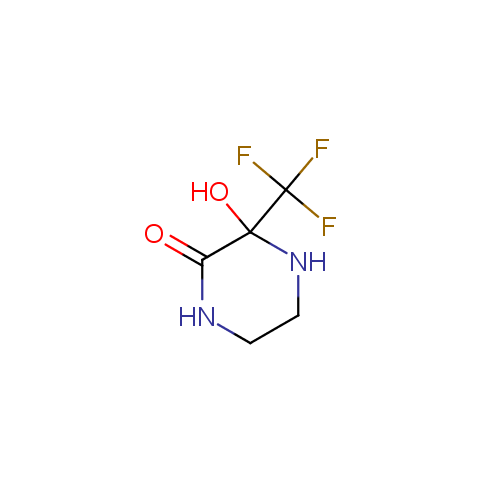
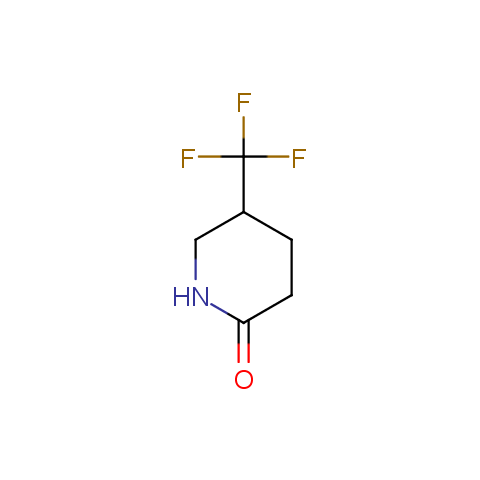
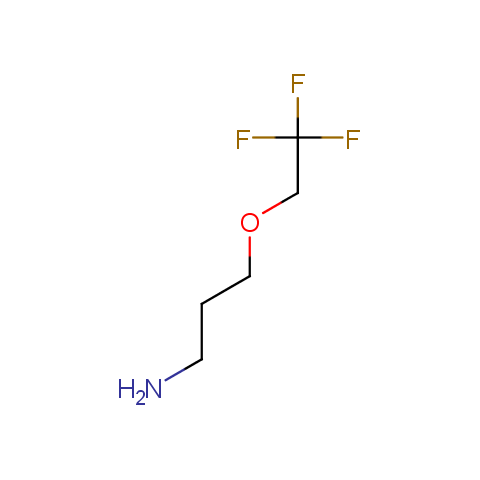
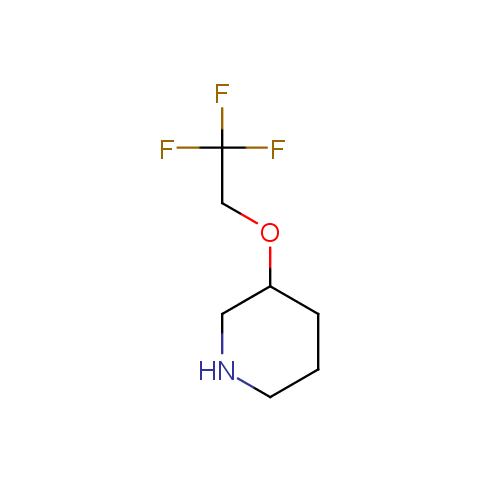
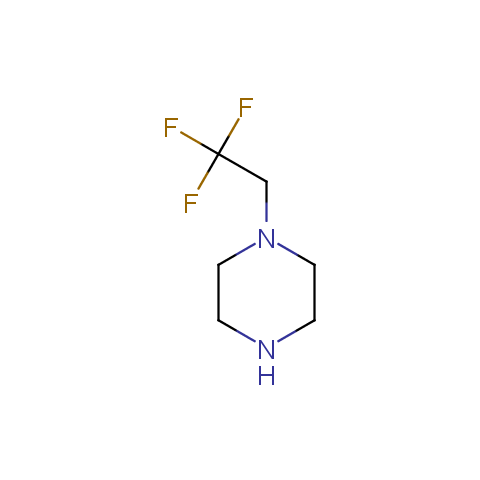
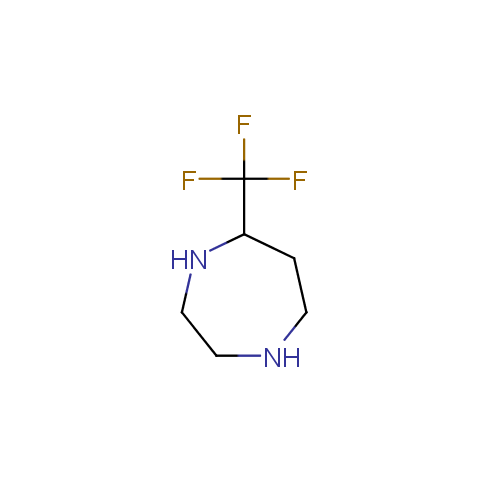
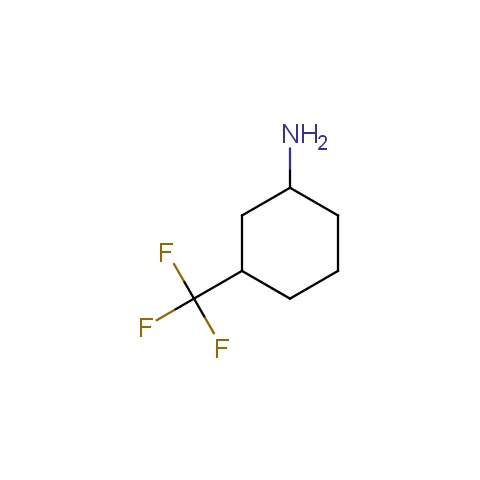
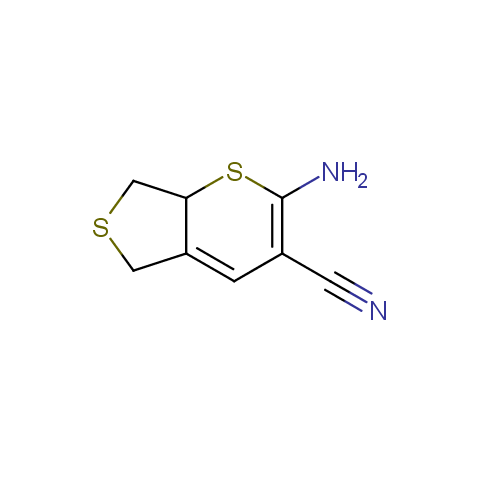
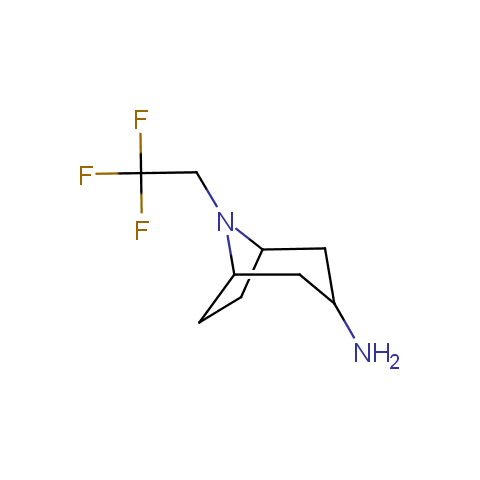
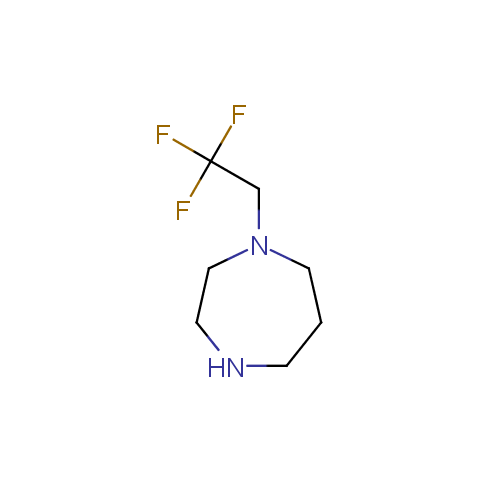
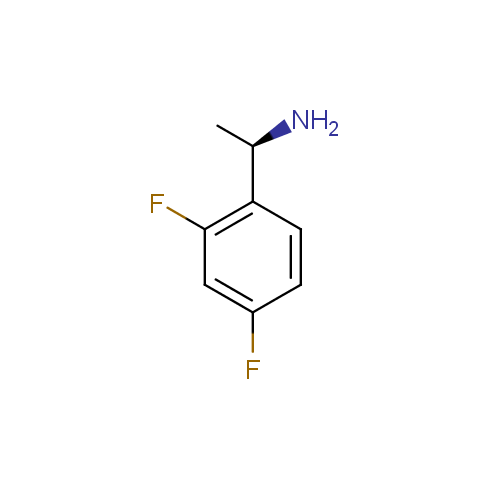
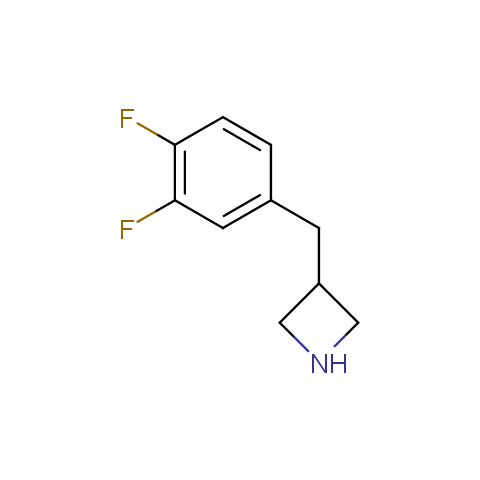
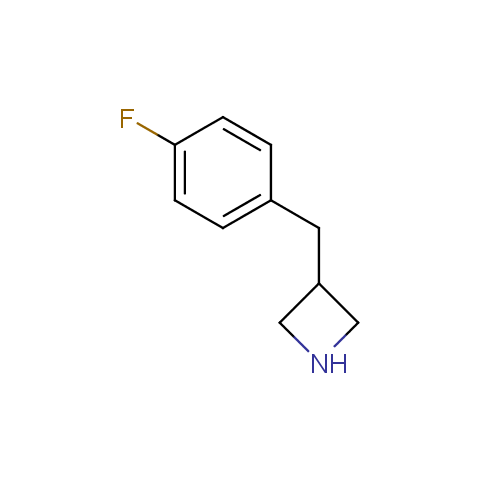
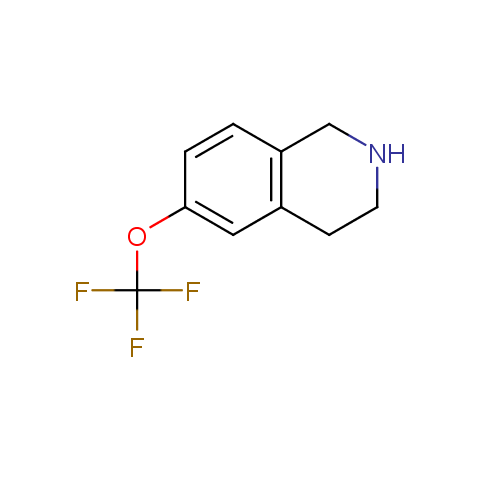
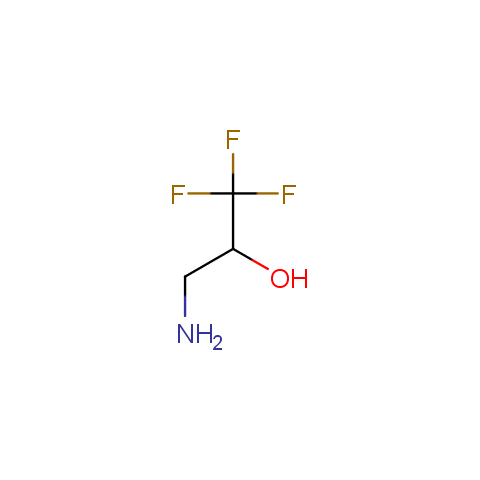
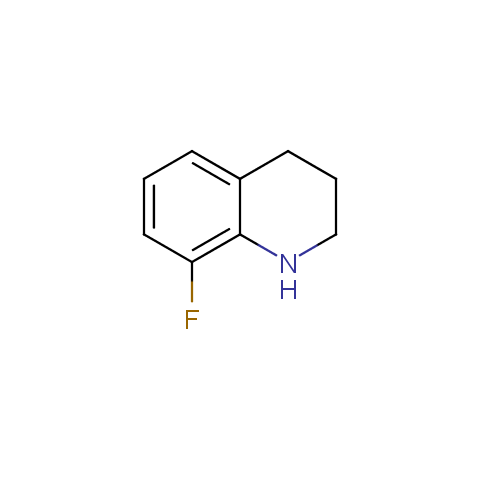
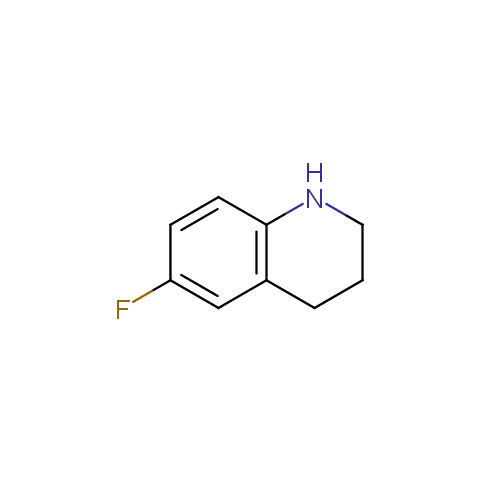
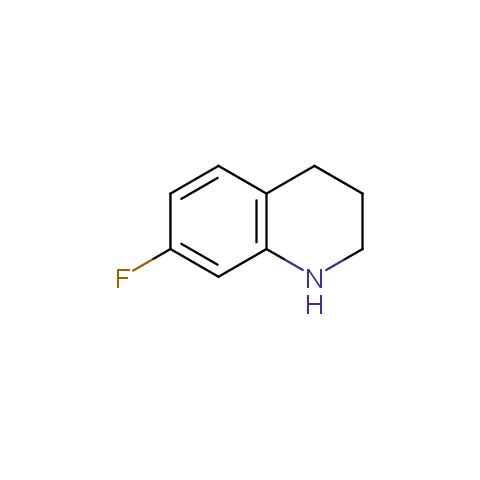
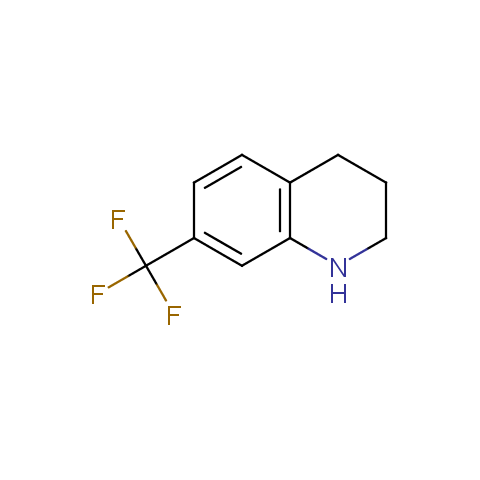
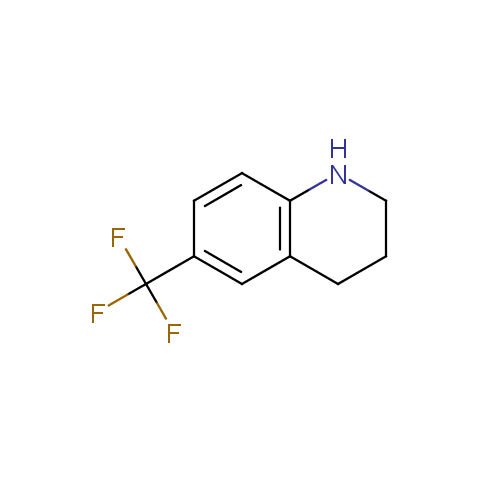
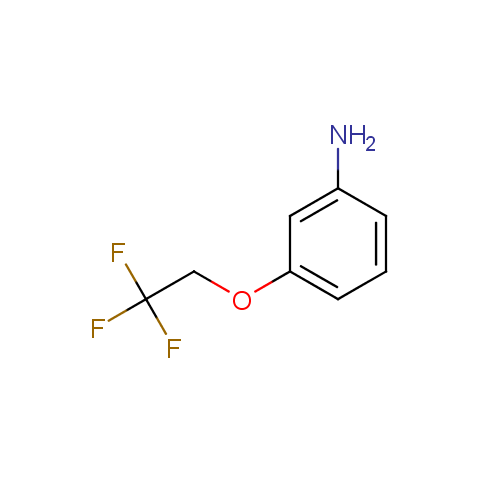
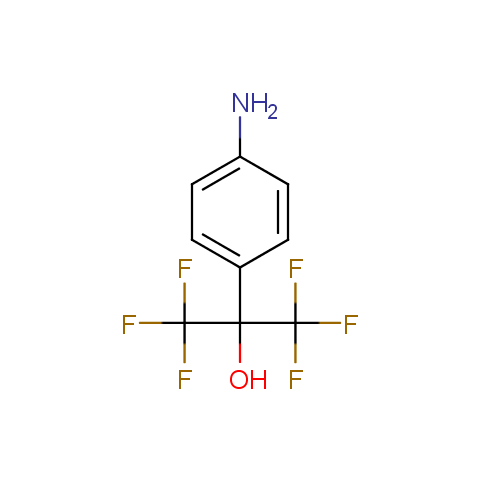
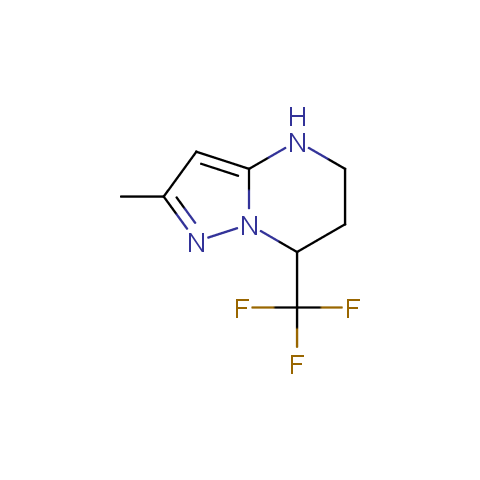
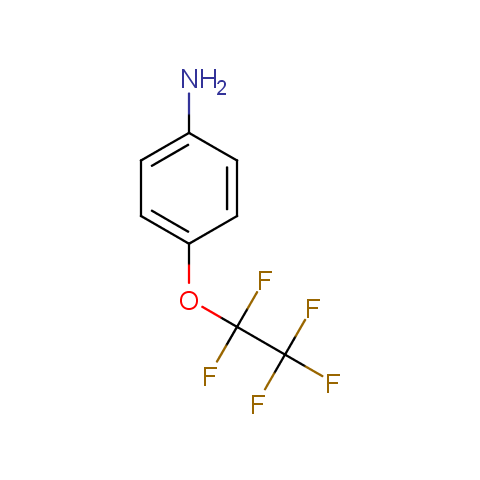
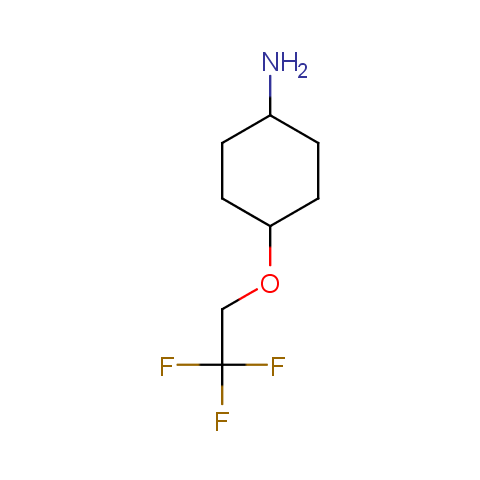
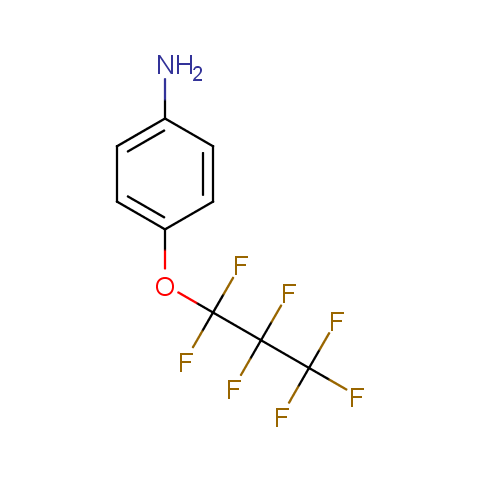
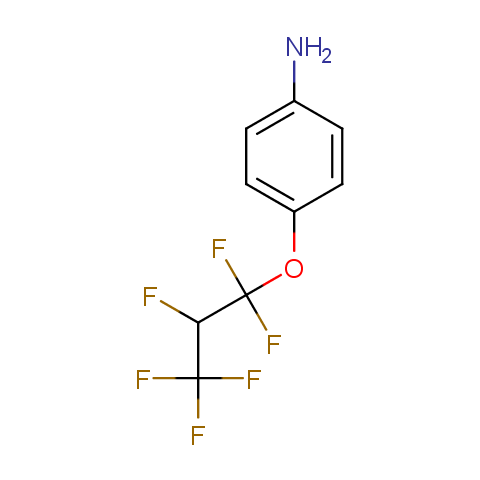
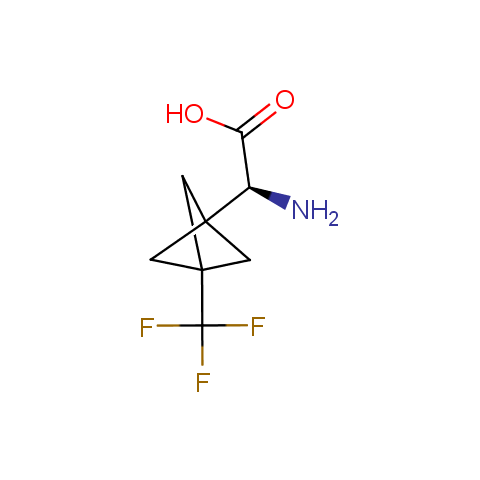
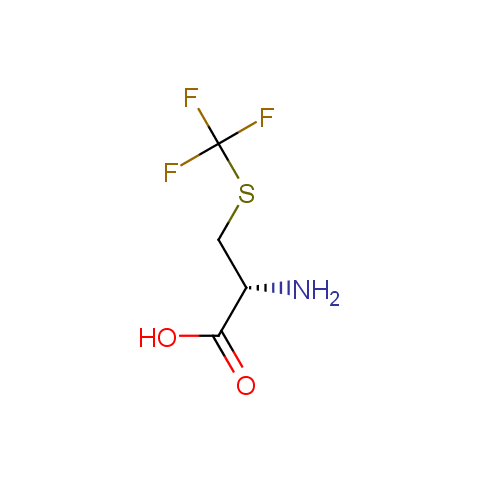
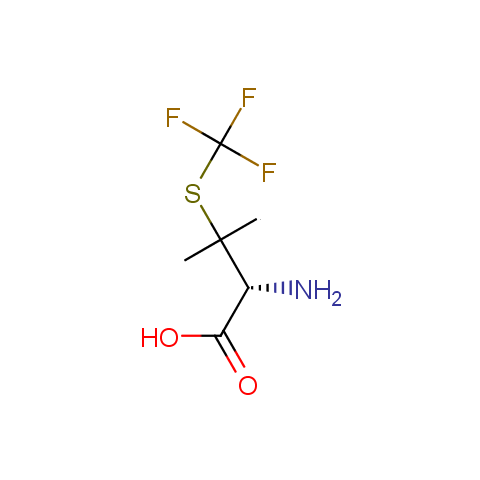
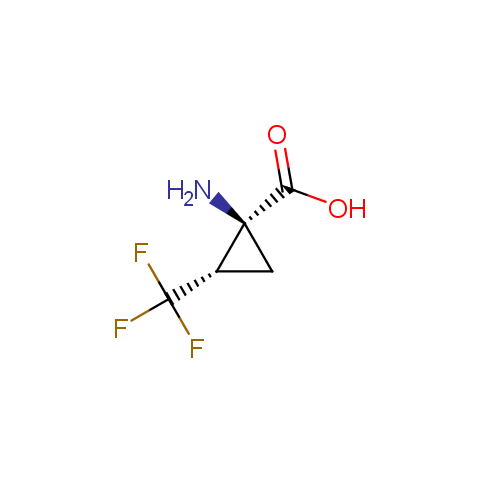
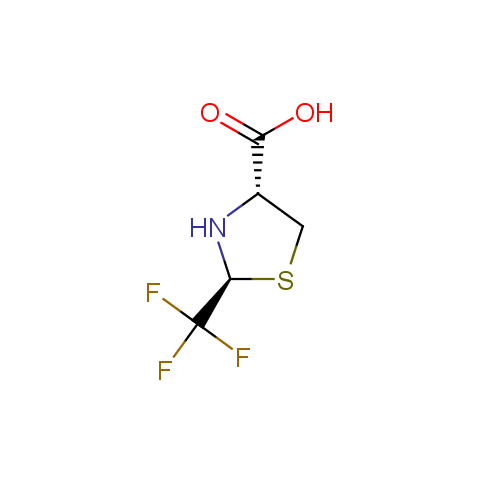
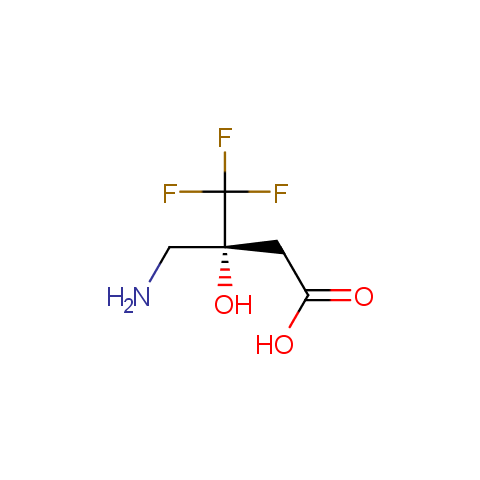
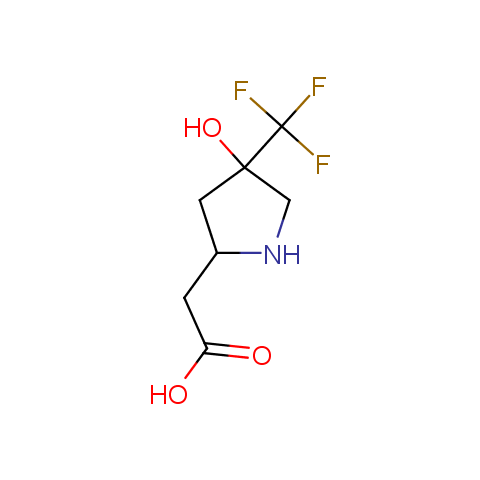
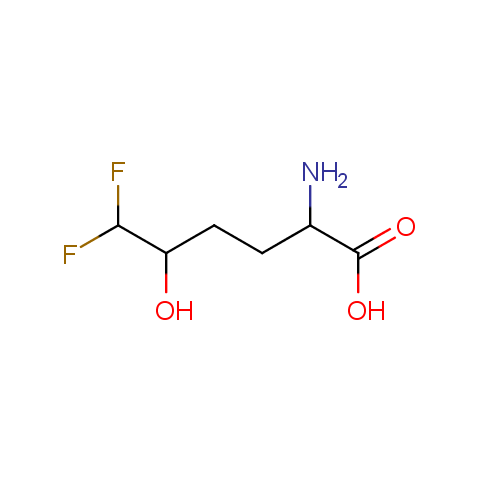
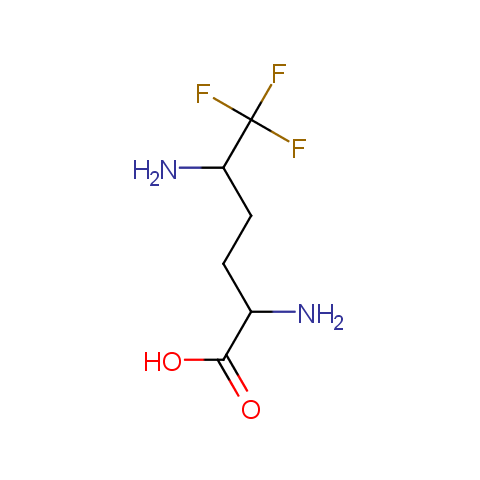
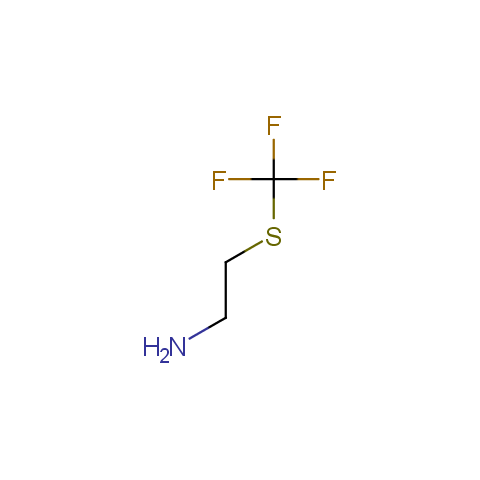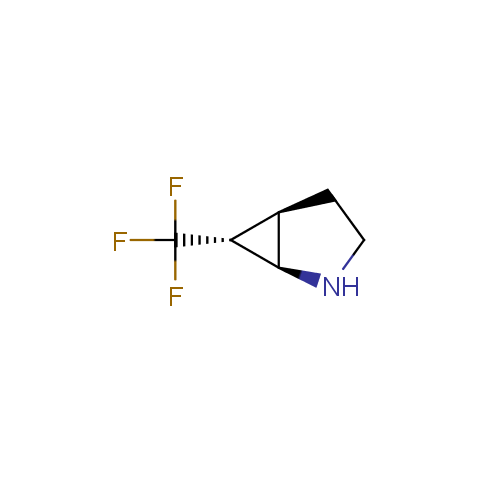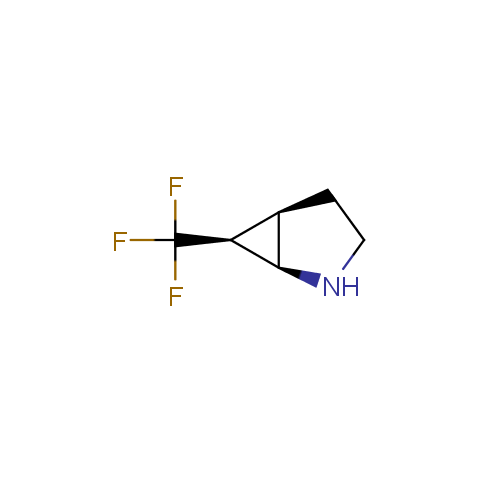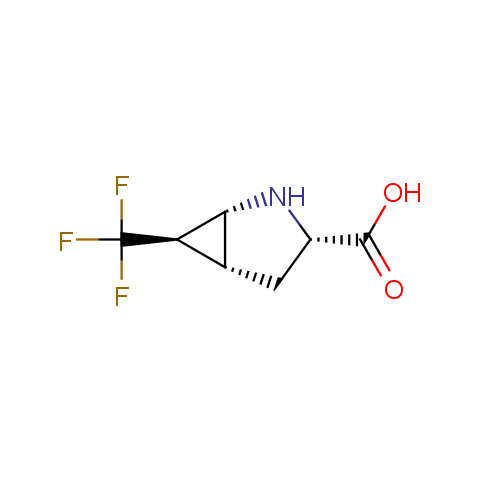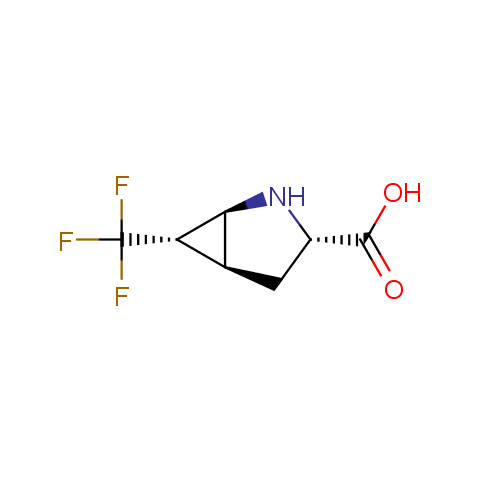In the previous issue, we presented the set of building blocks which had at least one fluorine substituent. This issue continues representation of fluorine-substituted building blocks emphasizing compounds derived from original scaffolds designed by Enamine chemists.
Understanding the importance of the fluoro-organic compounds for the pharmaceutical industry, we put much effort in adapting known and developing new synthetic methodologies allowing introducing fluorine by the use of different fluorine reagents.
Apart from classical perfluoroalkyl-containing synthones (e.g. Ethyl trifluoroacetate, Trifluoroacetaldehyde, 1,1,1-Trifluoroacetone, 1,1,1-Trifluoroacetylacetone, Ethyl 1,1,1-trifluoroacetoacetate etc.) the arsenal of the reagents includes:
- DAST, MorphDAST and Sulfur tetrafluoride;
- Hydrogen fluoride;
- Metal fluorides and TBAF;
- Tetrafluoroboric acid (for Schiemann process);
- Trifluorotrimethylsilane;
- Trifluoromethyl iodide;
- Trifluoromethyl diazomethane;
- Phenyl(thifluorometyl)mercury;
- Trifluoroacetonitrile;
- Perfluoropropene and its epoxide.
The compounds obtained in such way are original, in many cases unavailable at any other supplier. Many of them were obtained by stereoselective routes; therefore, they are enantiomerically pure or enriched. The procedures employing the reagents mentioned above allow obtaining a large diversity of original fluorine-containing building blocks at 1–10 g scale; novel compounds of the requested structure can be obtained in 4–8 weeks.
Below, we show some examples of the Original Fluorine-Substituted Building Block set.
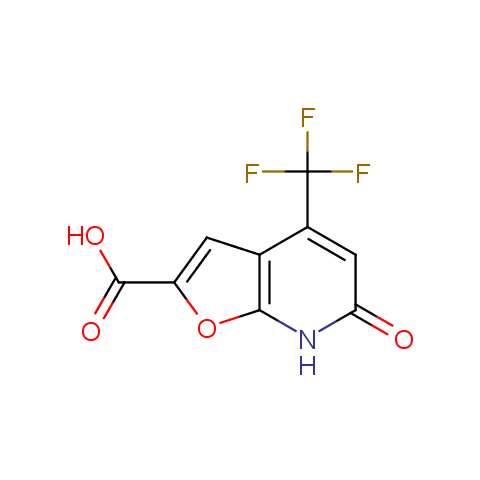
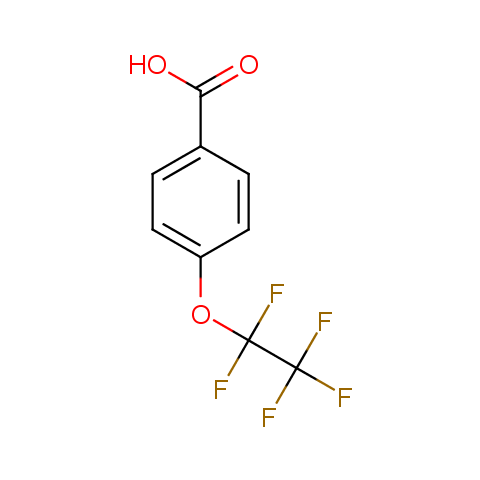
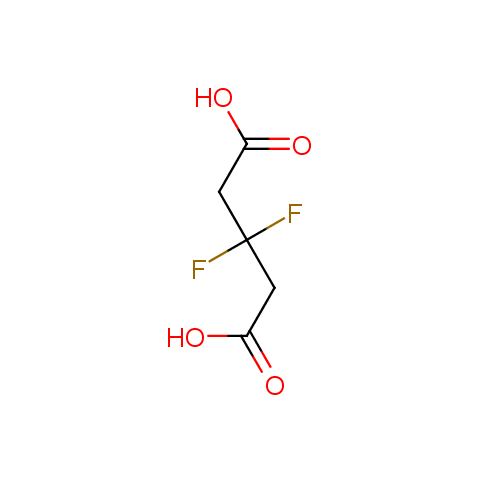
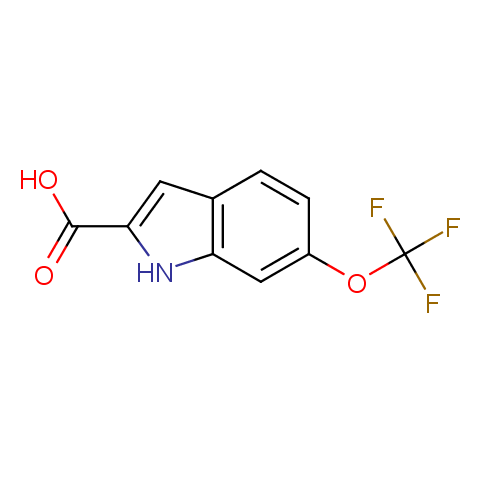
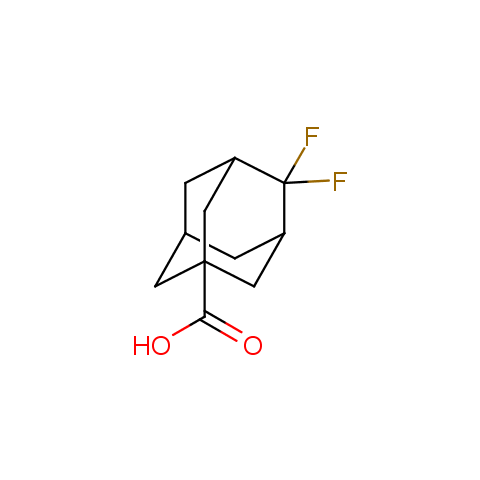
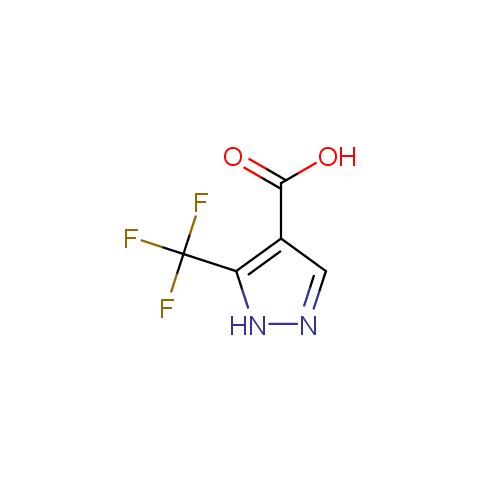
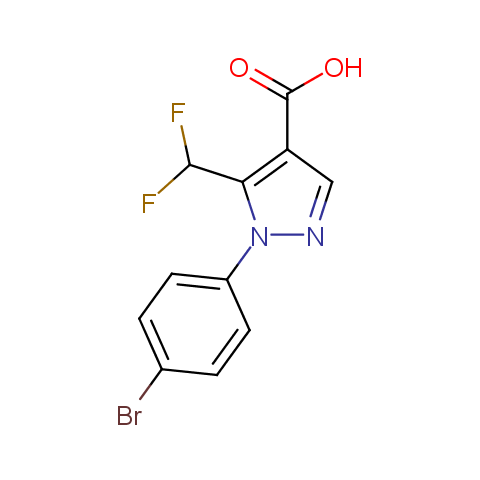
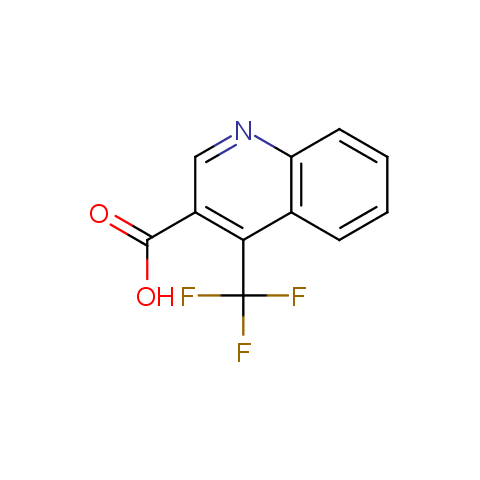
Carboxylic acids
Reactive aryl halogenides
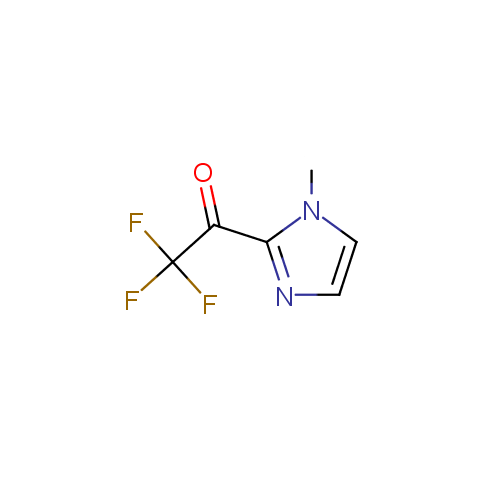
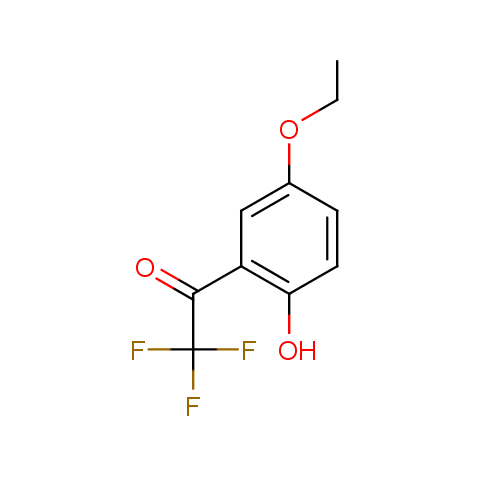
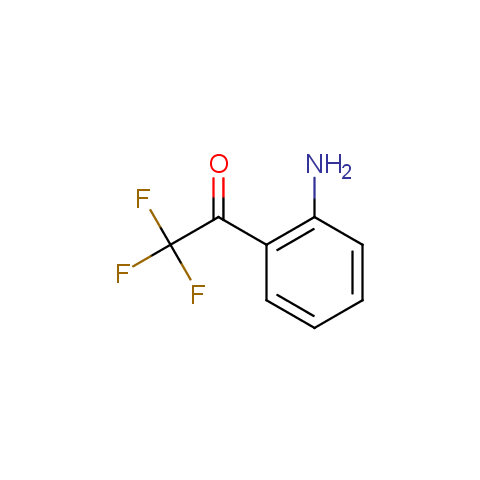
Trifluoromethyl ketones
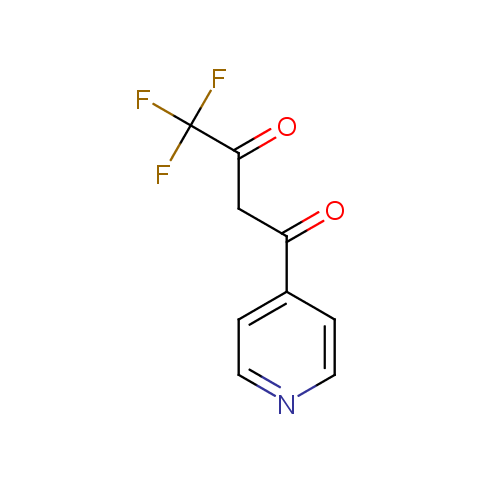
Trifluoromethyl β-dicarbonyl compounds
Cyclic amides
Aliphatic amines
Aromatic amines