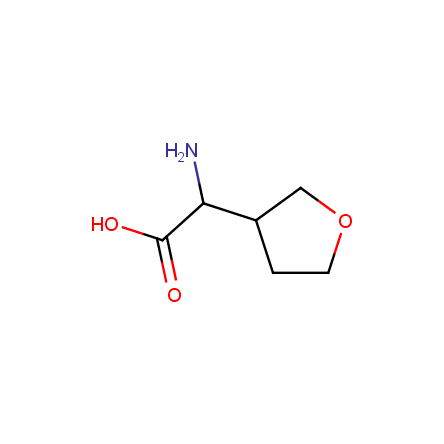
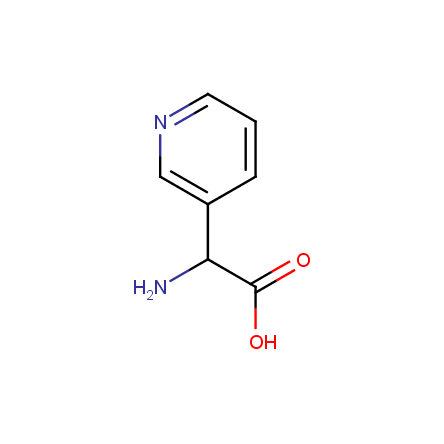
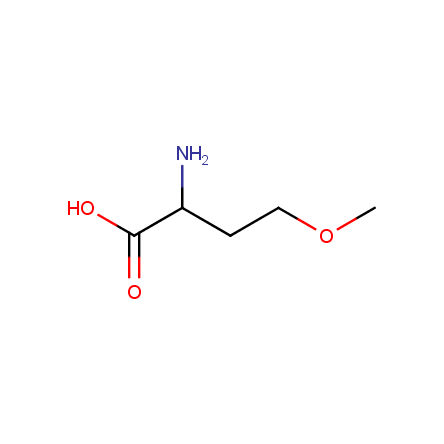
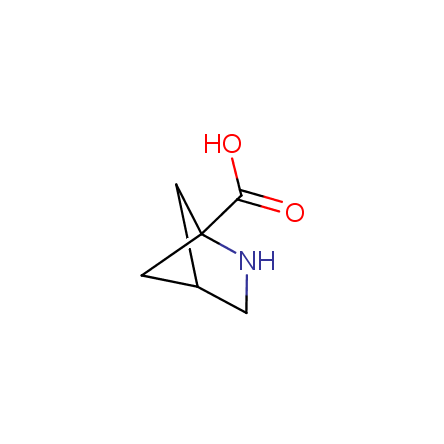
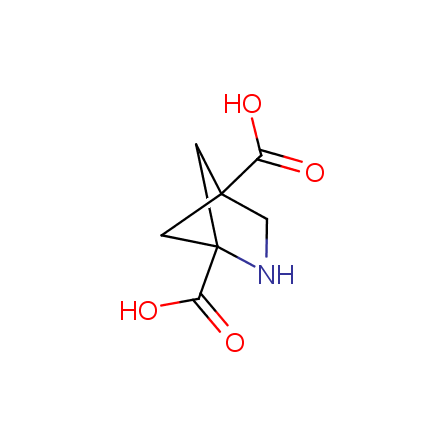
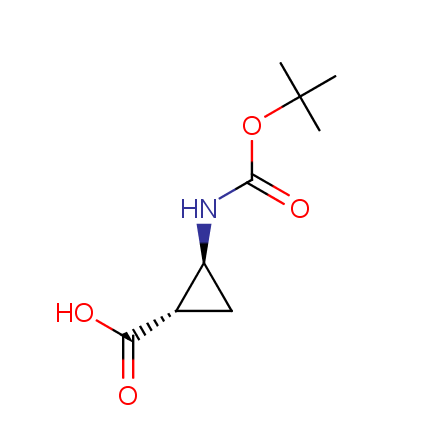
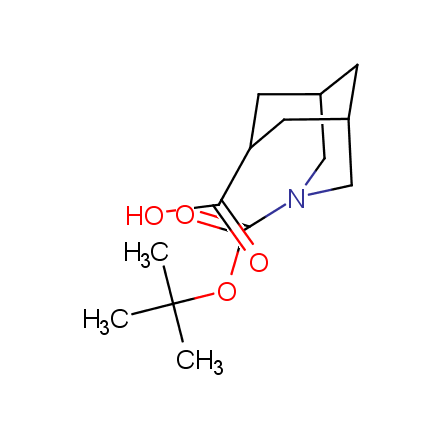
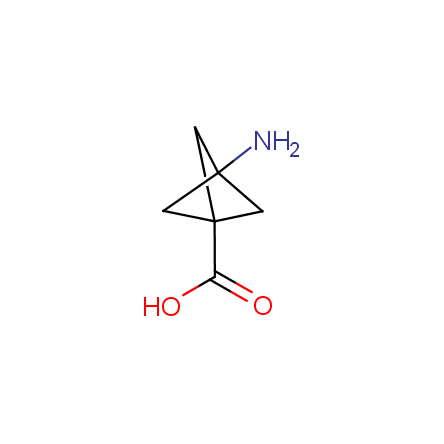
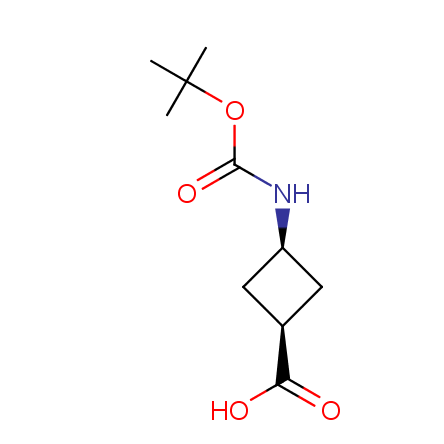
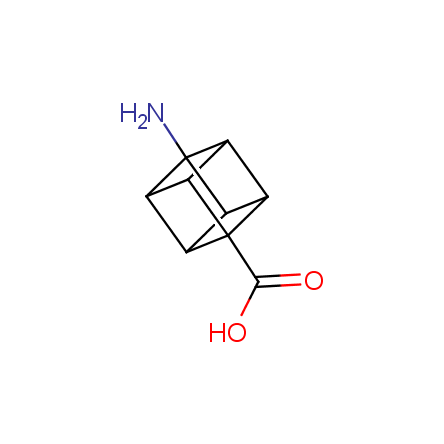
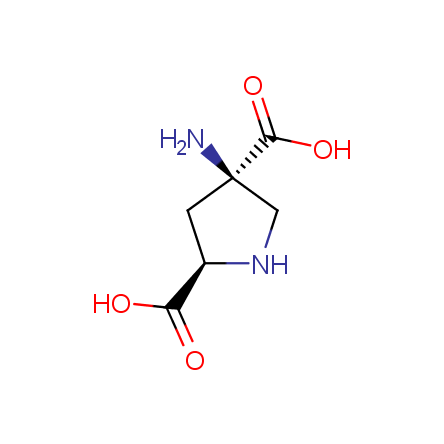
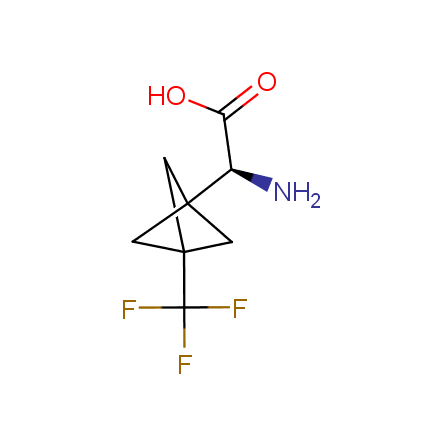
At present, many suppliers of the building blocks for drug design offer large selection of the unnatural amino acids. Enamine committed itself to create a library of the unusual amino acids which would differ from the available on the market. First, we offer a number of racemic α-amino acids at the very reasonable prices. The price category, together with extremely wide chemical diversity, makes these amino acids especially attractive in lead discovery by HTS. Examples illustrating chemical diversity of this set can be found below:
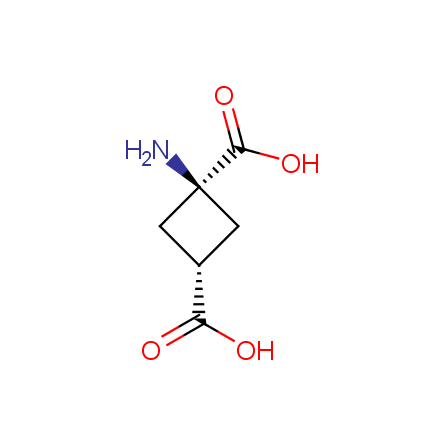
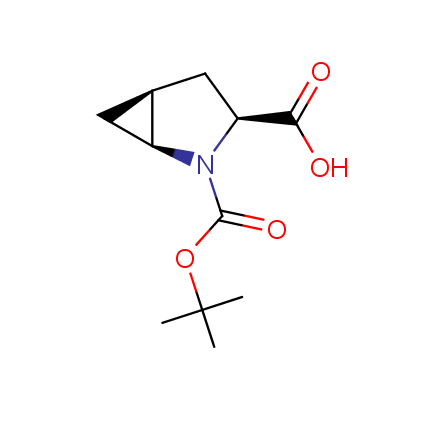
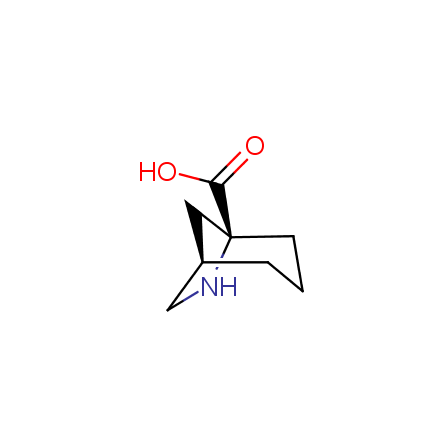
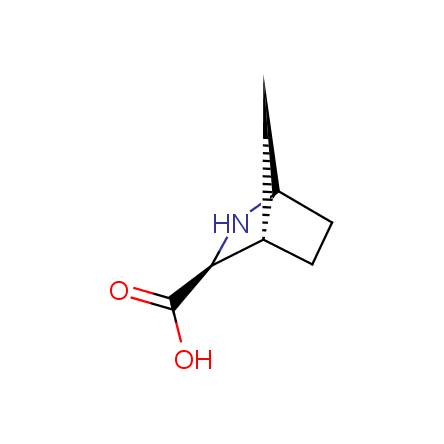
Other principles were used when compiling a set of conformationally restricted amino acids. The peptidomimetics obtained on the basis of conformationally restricted unnatural amino acids are expected to be biologically active due to their better preorganization resulting in stronger interaction with target biomolecules. These amino acids are optically pure (where applicable), and often can be supplied in both enantiomeric forms. N-protected derivatives, ready for the automated peptide synthesis are also available. Large selection of cyclic and polycyclic amino acids alows for variation of the geometry of the peptidomimetics in a vide range, fine-tuning the structure to the need of structure-based drug design. Low molecular mass and favourable lipophilicity make them useful in the fragment-based lead discovery. Some examples are shown below:
Yet another subset of the Enamine Amino Acid Selection Database consists of conformationally restricted β-, γ- and ω- amino acids. Their use in the peptidomimetic design has been shown to lead to extremely unusual structural consequences and promising biological activity, see, for example, and references therein. Here, too, chiral compounds are available as single enantiomers of high optical purity. In most cases, these amino acids are available in protected form ready for peptide synthesis.
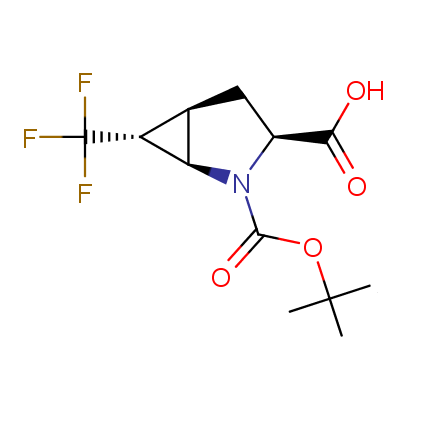
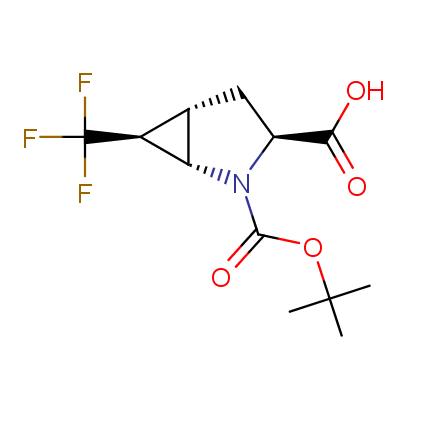
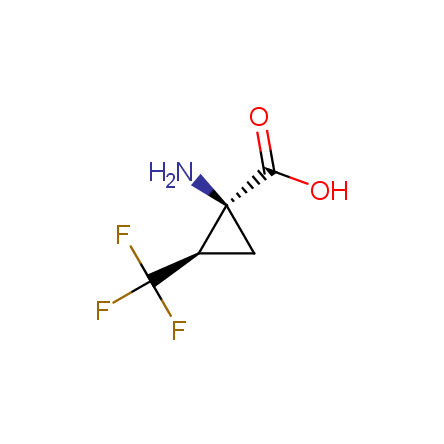
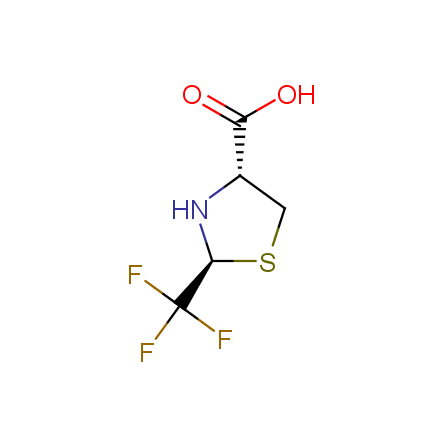
Finally, Enamine offers a vide variety of the fluorine-substituted α- and γ- amino acids. These compounds can be used in drug design and also as biomarkers, 19F labels in NMR-spectroscopy. In the synthesis of the fluorine-substituted amino acids our chemists use the experience in the fluorine chemistry acquired in cutting-edge research carried out at the Ukrainian scientific institutions. Examples: