Small library of specially synthesized compounds
320 compounds
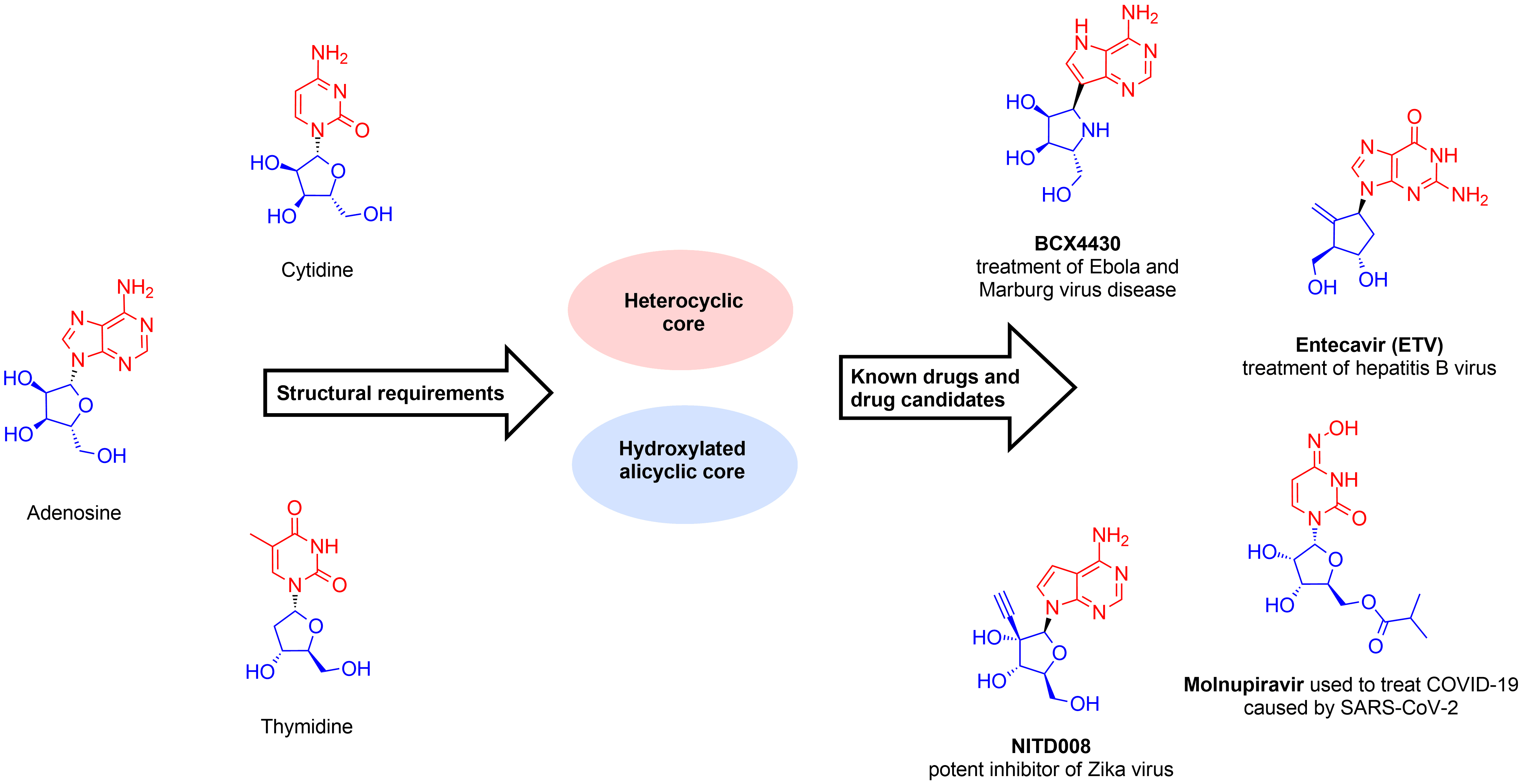
Mimetics of nucleosides are special class of proven therapeutic agents, which already showed high efficacy in treatment of viral infections, cancer and bacterial disease. In spite of similar structural motifs and common moieties and even cores, many modified nucleosides display high selectivity to certain class of targets. This type of molecules is well-tunable when using at least two sites of possible modification. Recently, we developed few alternative parallel chemistry approaches to make these compounds more accessible within reasonable time and in cost-effective manner. Using our library for your hit finding you receive multiple benefits:
- Hit Confirmation support – resupply from dry samples, QC check, HPLC repurifiction, guaranteed resynthesis.
- Straightforward synthesis of follow-up libraries through our REAL Database technology.
- Medicinal chemistry support enhanced with on-site broad ADME panel.
Typical Formats
Nucleoside Mimetics Library is available for supply in various pre-plated formats, including the following most popular ones:
Catalog No.
NML-320-0-Z-10
Compounds
320
1 plate
Amount
≤ 300 nL of 10 mM of DMSO solutions
Plates and formats
1536-well Echo LDV microplates, first and last four columns empty, 1280 compounds per plate
Price
Catalog No.
NML-320-10-Y-10
Compounds
320
1 plate
Amount
≤ 10 µL of 10 mM DMSO solutions
Plates and formats
384-well, Echo Qualified LDV microplates #001-12782 (LP-0200), first and last two columns empty, 320 compounds per plate
Price
Catalog No.
NML-320-50-Y-10
Compounds
320
1 plate
Amount
50 μL of 10 mM DMSO solutions
Plates and formats
384-well, Greiner Bio-One plates #781280, first and last two columns empty, 320 compounds per plate
Price
Catalog No.
Library & follow-up package
Plates and formats
NML-320-10-Y-10 screening library 320 cmpds, hit resupply, analogs from 4.7M+ stock and synthesis from REAL Space
Price
*We will be happy to provide our library in any other most convenient for your project format. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400), C52621 or send your preferred labware. Compounds pooling can be provided upon request.
Download SD file
Library design
- Careful Substructure Search Search of nucleoside core fragments and their bioisosteric heterocycles with simultaneous presence of sugar-like moiety
- Strict MedChem Filters Removal of undesired functionalities, reactive groups and large aromatic fragments
- Chemotype Control Compounds with trivial structural fragments were mostly removed. Control of renovation of the library in accordance to new literature data.
Design of REAL Nucleoside mimetics dataset
REAL structures with validated synthetic procedures
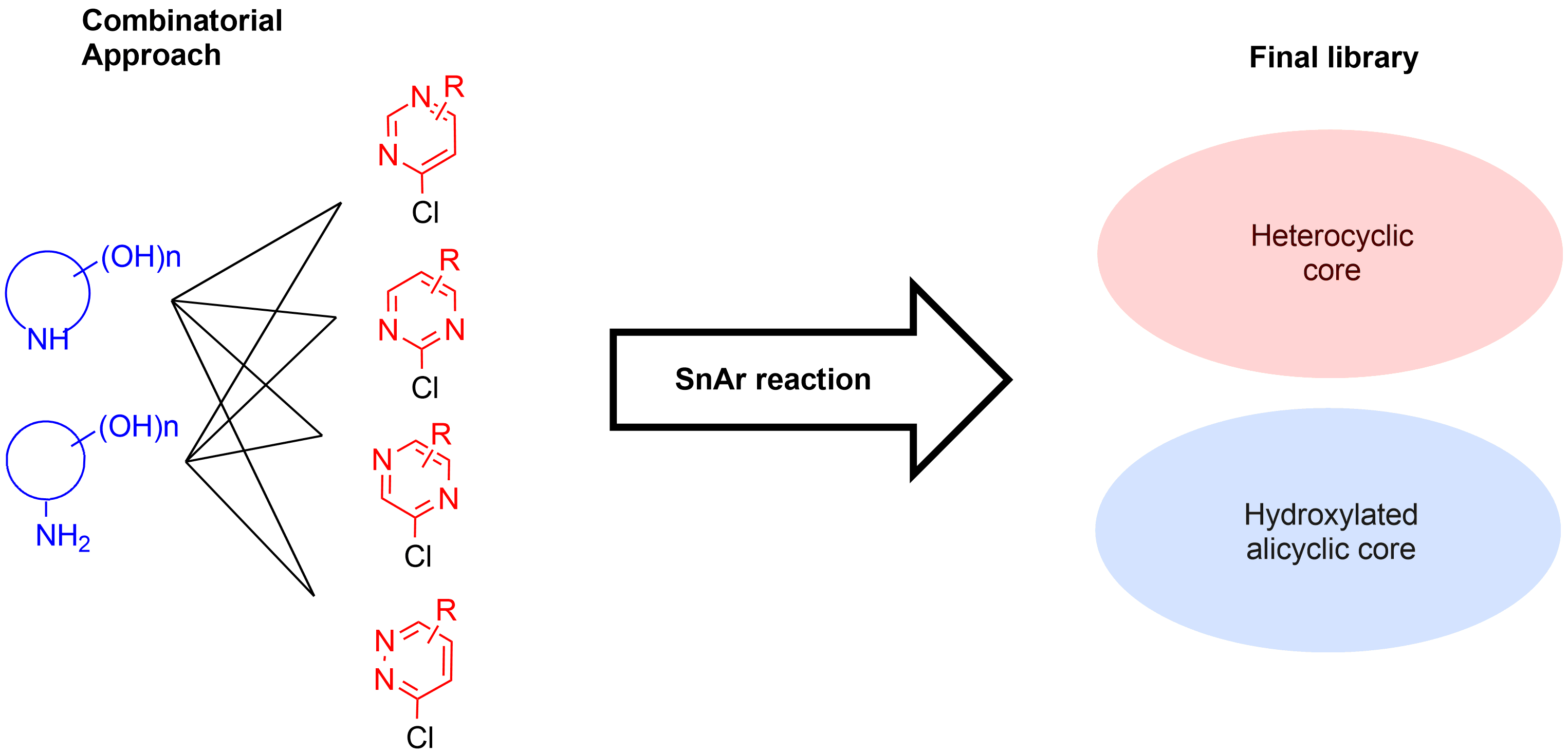
- Combinatorial approach Over 100 000 Building Blocks immediately available for modification:
- Diazine heterocyclic cores mimic nucleobases
- alicyclic amino alcohols - sugars
- Filtering of unwanted functionalities & MedChem inspection
- Automated parallel synthesis Implementation of well-developed reaction procedures operated by highly experienced staff
Design of REAL Nucleoside mimetics dataset