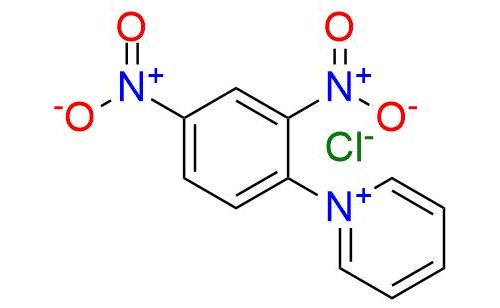
CAS 4185-69-7, Cat. No EN300-45333
Reagent for synthesis of N-pyridinium salts and Zincke aldehydes
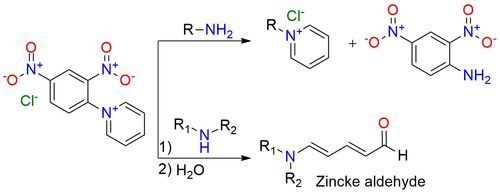
1-(2,4-Dinitrophenyl)pyridinium chloride (Zincke salt) is a unique electrophilic reagent that enables the synthesis of pyridinium salts with primary amines1. The compound is a white, stable solid with solubility in protic and other highly polar solvents. In cases where improved solubility in organic solvents is desired, it can be enhanced via anion exchange with a simple salt. The reaction involving electrophilic Zincke salts and nucleophilic primary alkyl- or arylamines leads to pyridinium salts, accompanied by the expulsion of 2,4-dinitroaniline. In many instances, this chemical transformation can undergo a one-pot procedure, in some cases, protocols may require the isolation of intermediate acyclic imines, followed by subsequent heating with an acid or base to induce cyclization. The reaction with secondary amines facilitates the formation of Zincke aldehydes, important building blocks.
Synonyms: 1-(2,4-dinitrophenyl)pyridinium chloride (6CI, 7CI); pyridinium, 1-(2,4-dinitrophenyl)-, chloride (8CI, 9CI); (2,4-dinitrophenyl)pyridinium chloride; 1-(2,4-dinitrophenyl)-1λ5-pyridin-1-ylium chloride; 1-(2,4-dinitrophenyl)pyridin-1-ium chloride; N-(2,4-dinitrophenyl)pyridinium chloride
Selected publication
-
1-(2,4-Dinitrophenyl)Pyridinium Chloride.
Lam J.; Vanderwal C. Encyclopedia of Reagents for Organic Synthesis 2014, 1–6. DOI: 10.1002/047084289X.rn01711