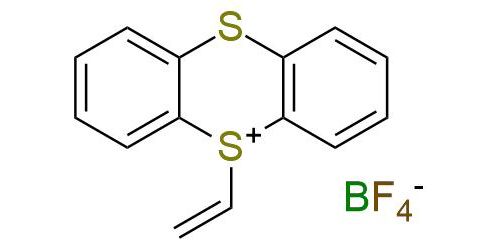
CAS 2703016-98-0, Cat. No EN300-43394895
Reagent for vinylation, labeling, cyclopropanation, and aziridination
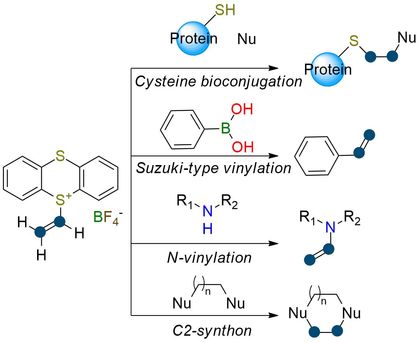
Vinylthianthrenium (Vinyl-TT+) tetrafluoroborate is an innovative vinylating reagent. Overcoming the challenges associated with incorporating a vinyl group as a C-2 building block, this reagent offers unique properties and reactivity profiles. With its broad substrate scope, Vinyl-TT+ tetrafluoroborate enables effective transformations, including N-vinylation of heterocycles, aryl boronic acids vinylation, and annulation of cyclic and heterocyclic substrates1. Reagent’s distinctive structure positions it as a superior performer in cross-coupling reactions. Moreover, Vinyl-TT+ tetrafluoroborate facilitates metal-free cyclopropanation and aziridination of alkenes with R-XH2 (X=N, C)2. The reagent reacts with bioorthogonal nucleophiles for site-selective protein labeling, cross-linking, and peptide stapling. This reaction allows the introduction of IR labels, NMR probes, and affinity tags in a single step, with fast reaction kinetics and high selectivity3.
Synonyms: 5-ethenyl-5H-thianthren-5-ium, tetrafluoroboranuide; 5-vinyl-5H-thianthren-5-ium tetrafluoroborate; 5-vinylthianthrenium tetrafluoroborate; Vinyl-TT+ tetrafluoroborate
Selected publications
-
Vinyl Thianthrenium Tetrafluoroborate: A Practical and Versatile Vinylating Reagent Made from Ethylene.
Juliá F.; Yan J.; Paulus F.; Ritter T. J Am Chem Soc 2021, 143 (33), 12992–12998. DOI: 10.1021/jacs.1c06632
-
Intermolecular Metal‐Free Cyclopropanation and Aziridination of Alkenes with XH2 (X=N, C) by Thianthrenation.
Liu M.; Du H.; Cui J.; Shu W. Angewandte Chemie International Edition 2022, 61 (41). DOI: 10.1002/anie.202209929
-
Chemoselective Umpolung of Thiols to Episulfoniums for Cysteine Bioconjugation.
Hartmann P.; Bohdan K.; Hommrich M.; Juliá F.; Vogelsang L.; Eirich J.; Zangl R.; Farès C.; Jacobs J.; Mukhopadhyay D.; Mengeler J.; Vetere A.; Sterling M.; Hinrichs H.; Becker S.; Morgner N.; Schrader W.; Finkemeier I.; Dietz K.; Griesinger C.; Ritter T. Nat Chem 2024, 16 (3), 380–388. DOI: 10.1038/s41557-023-01388-7