CAS 87-90-1, Cat. No EN300-120673
Reagent for chlorination and oxidation
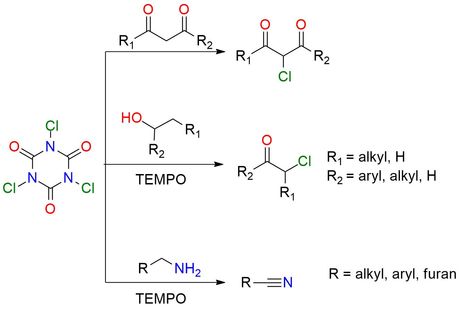
Trichloroisocyanuric acid (TCCA) is a versatile and powerful reagent in chlorination and oxidation reactions. It is more reactive than other N-chloro compounds and can be used instead of chlorine. TCCA is a stable, safe, white granular solid soluble in acetonitrile, acetone, acetic acid, and ethyl acetate and less soluble in benzene, chloroform, methylene chloride, and water1. The reagent is used for chlorinating aromatics, amines, amides, and carbonyl compounds2. Also, it can be used to oxidize amines to nitriles and alcohols to corresponding ketones, and aldehydes. Generally, the reactions proceed in mild conditions and do not require special precautions. The good solubility of TCCA and the cyanuric acid formation as a byproduct, that is easy to remove by filtration, make it one of the best reagents for chlorination2.
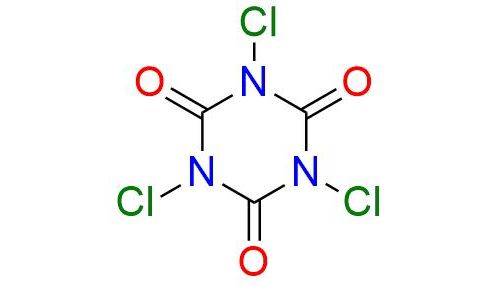
Synonyms: TCCA; TCICA; 1,3,5-trichloro-1,3,5-triazine-2,4,6 (1H,3H,5H)-trione; trichloro-s-triazinetrione; isocyanuric chloride
Selected publications
-
Trichloroisocyanuric Acid.
Hiegel G.; Pozzi G.; AnuMahadevan Encyclopedia of Reagents for Organic Synthesis 2013. DOI: 10.1002/047084289X.rt209.pub3
-
Trichloroisocyanuric Acid: A Versatile and Efficient Chlorinating and Oxidizing Reagent.
Gaspa S.; Carraro M.; Pisano L.; Porcheddu A.; De Luca L. European J Org Chem 2019, 2019 (22), 3544–3552. DOI: 10.1002/ejoc.201900449