CAS 140681-55-6, Cat. No EN300-98145
Reagent for electrophilic fluorination
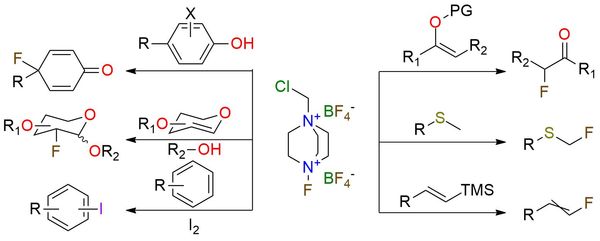
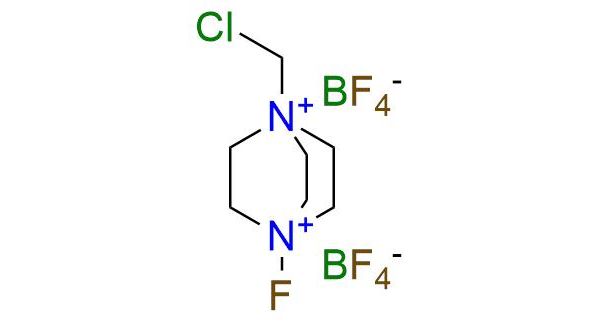
1-(Chloromethyl)-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (F-TEDA-BF4 or Selectfluor™) is a highly stable and practically nonhygroscopic crystalline solid, representing a significant advancement in electrophilic fluorinating agents. Unlike traditional reagents that require special handling and pose potential hazards, F-TEDA-BF4 offers exceptional safety and ease of use1. Being a dication, the reagent is soluble in a few polar solvents, including acetonitrile, DMF, water, and nitromethane. The versatility of F-TEDA-BF4 is remarkable, with a wide scope of applications. It serves as a powerful electrophilic fluorine donor, enabling α-fluorination of carbonyl functionalities, as well as the fluorination and oxidation of thioethers2. Additionally, it plays a key role in synthesizing glycosyl fluorides and the fluorination of alkenes and aromatic compounds. Moreover, Selectfluor™ showcases oxidation capabilities, allowing for the oxidation of benzylic alcohols and tertiary carbon centers1,2. The broad range of applications makes F-TEDA-BF4 an indispensable reagent and a universal tool in synthetic chemistry.
Synonyms: 1-(chloromethyl)-4-fluoro-1,4-diazabicyclo[2.2.2]octane-1,4-diium, bis(tetrafluoroboranuide); 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate); 1-(chloromethyl)-4-fluoro-1,4-diazabicyclo[2.2.2]octane-1,4-diium tetrafluoroborate; F-TEDA-BF4; Selectfluor™
Selected publication
-
1-(Chloromethyl)-4-Fluoro-1,4-Diazoniabicyclo[2.2.2]Octane Bis(Tetrafluoroborate).
Banks R.; Murtagh V.; An I.; Maleczka R. Encyclopedia of Reagents for Organic Synthesis 2007. DOI: 10.1002/047084289X.rc116.pub2
-
Achievements in Fluorination Using Variable Reagents through a Deoxyfluorination Reaction.
Aggarwal T.; Sushmita; Verma A. Organic Chemistry Frontiers 2021, 8 (22), 6452–6468. DOI: 10.1039/D1QO00952D