CAS 37342-97-5, Cat. No EN300-53119656
Organozirconium reducing reagent
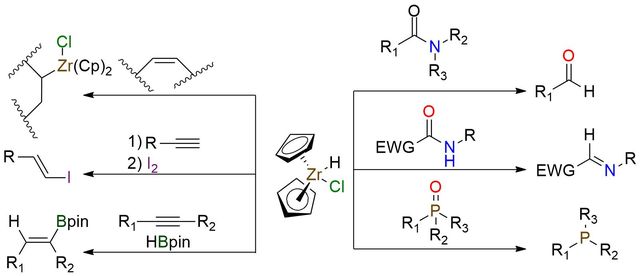
Schwartz's reagent is often a starting point for many organozirconium complexes and can be applied in various reduction reactions1. It is a white bench-stable powder insoluble in organic solvents but photosensitive. The reagent is used to synthesize many organozirconium compounds from alkenes and alkynes. Hydrozirconation of alkenes places the zirconium moiety at the sterically least hindered position. For the alkynes, Zr–H addition proceeds with cis stereochemistry. An alternative application of the reagent is a reduction process with unique properties. It includes the efficient reduction of phosphine oxides, secondary amides, tertiary amides to aldehydes (with tolerated carbonyl group), and lactams to N-substituted imines. Schwartz's reagent can be used as a catalyst in hydroboration reactions with internal and terminal alkynes. Numerous reactions that use Schwartz's reagent are described in the literature. It makes this organozirconium compound an excellent addition to the arsenal of synthetic approaches.
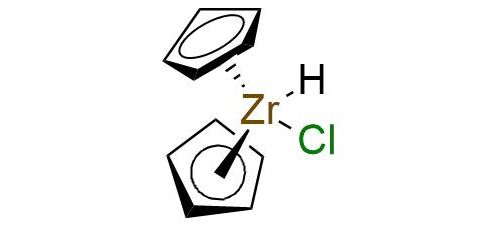
Synonyms: chlorobis(cyclopentadienyl)hydridozirconium; zirconocene chloride hydride; bis(cyclopentadienyl)zirconium(IV) chloride hydride; Schwartz's reagent
Selected publication
-
Chlorobis(Cyclopentadienyl)Hydridozirconium.
Takahashi T.; Suzuki N.; Jayasuriya N.; Wipf P. Encyclopedia of Reagents for Organic Synthesis 2006. DOI: 10.1002/047084289X.rc074.pub2