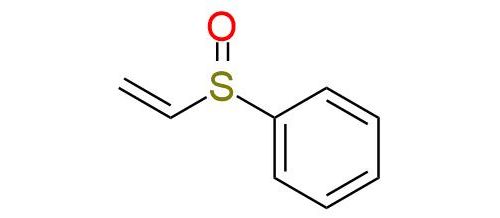
CAS 20451-53-0, Cat. No EN300-7407239
Acetylene equivalent reagent
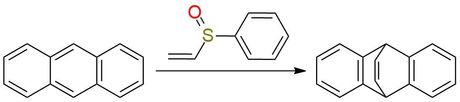
Phenyl vinyl sulfoxide is a colorless liquid used in various organic reactions, including Diels-Alder, dipolar cycloadditions, and Michael additions1. It acts as an acetylene equivalent and is the only reagent of this type able to lose the activating functionality spontaneously after the cycloaddition. The reagent can be deprotonated to form an α-sulfinyl carbanion for other chemical transformations. Phenyl vinyl sulfoxide is involved in the synthesis of complex heterocycles, such as nicotyrines and indolizines. Moreover, it can be employed in Pummerer reactions or transition metal-mediated processes such as Heck coupling and allylic C-H oxidation reactions.
Synonyms: phenylsulfinylethylene; vinylsulfinyl-benzene; ethenesulfinyl-benzene; (ethenylsulfinyl)benzene; phenyl vinyl sulphoxide
Selected publication
-
Phenylsulfinylethylene.
De Lucchi O.; Licini G.; Krasnova L.; Yudin A. Encyclopedia of Reagents for Organic Synthesis 2008. DOI: 10.1002/047084289X.rp103.pub2