CAS 14338-32-0, Cat. No EN300-21556
Reagent for carboxylic acid activation
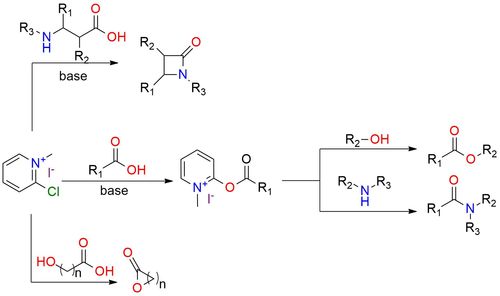
Mukaiyama reagent is a versatile tool in carboxylic acids chemistry, that facilitates the formation of esters and amides, lactones, ketene, and β-lactams1,2. It is a hygroscopic yellow solid, bench-stable that is easy to work with. The reagent is soluble in most organic solvents, but the best for the reaction is DCM1. The reason is that the co-product from the reaction is insoluble in DCM and precipitates from the solvent2. Good results are obtained even for hindered carboxylic acids and alcohols. Analogous, the reaction works well for amide synthesis1. This method can be used for, lactones and β-lactams synthesis. If a carboxylic acid contains a suitably placed alkene, the resulting ketene can undergo intramolecular [2 + 2] cycloaddition to yield ketene1.
Synonyms: 2-chloro-1-methylpyridinium iodide
Selected publication
-
2-Chloro-1-Methylpyridinium Iodide.
Armstrong A.; Wang Y.; Wang P. Encyclopedia of Reagents for Organic Synthesis 2008. DOI: 10.1002/047084289X.rc126.pub2
-
A Convenient Method for the Synthesis of Carboxylic Esters.
Mukaiyama T.; Usui M.; Shimada E.; Saigo K. Chem Lett 1975, 4 (10), 1045–1048. DOI: 10.1246/cl.1975.1045