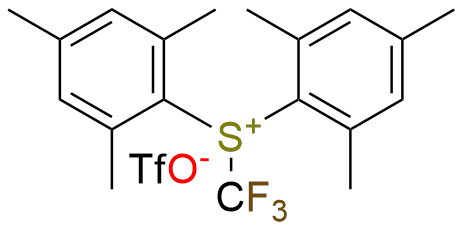
CAS 1895006-01-5, Cat. No EN300-22891572
Reagent for trifluoromethylation and arylation
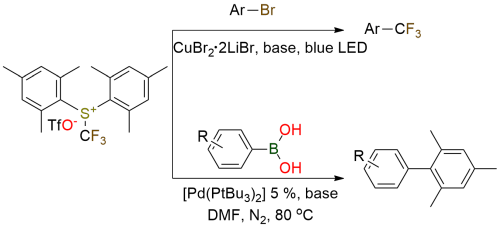
Mes-Umemoto reagent is a well-known source of electrophilic trifluoromethyl group. Many developed trifluoromethylation or C-C bond formation methodologies can be employed for various substrates. Copper-catalyzed trifluoromethylation of alkyl bromides[1],[2], and palladium-catalyzed arylation of arylboronic acids are among them[3]. Copper-catalyzed trifluoromethylation demonstrates broad compatibility with diverse functional groups, including alcohols, esters, amides, and protected amines that can be employed for late-stage functionalization. It’s effective for primary and secondary cyclic alkyl bromides, heterocycles, allylic, benzylic bromides, and heteroarenes[1],[2]. Palladium-catalyzed arylation works well with electron-rich and electron-poor arylboronic acids and provides the corresponding arylation products in good to high yields[3].
Synonyms: Dimesityl(trifluoromethyl)sulfonium trifluoromethanesulfonate; (trifluoromethyl)bis(2,4,6-trimethylphenyl)sulfanium triflate
Selected publication
-
Copper-Catalyzed Trifluoromethylation of Alkyl Bromides.
Kornfilt D. J. P.; Macmillan D. W. C. J Am Chem Soc 2019, 141 (17), 6853–6858. DOI: 10.1021/jacs.9b03024
-
A Radical Approach to the Copper Oxidative Addition Problem: Trifluoromethylation of Bromoarenes.
Le C.; Chen T. Q.; Liang T.; Zhang P.; Macmillan D. W. C. Science 2018, 360 (6392), 1010-1014. DOI: 10.1126/science.aat4133
-
Palladium-Catalyzed Arylation of Arylboronic Acids with Yagupolskii-Umemoto Reagents.
Wang S. M.; Wang X. Y.; Qin H. L.; Zhang C. P. Chemistry - A European Journal 2016, 22 (19), 6542–6546. DOI: 10.1002/chem.201600991