CAS 19172-47-5, Cat. No EN300-26784
Reagent for thionation of carbonyl compounds
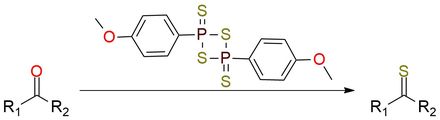
Lawesson’s reagent is a classical way to convert carbonyl group into thiocarbonyls1. It is a yellowish bad-smelling crystals, modestly soluble in boiling organic solvents such as toluene, chlorobenzene, anisole, and dimethoxyethane. Ketones, enones, carboxylic esters, thiolocarboxylic esters, amides, and related substrates are conveniently transformed into the corresponding thiocarbonyl compounds with good yields. A carbon-oxygen single bond also can be changed into a carbon-sulfur single bond using Lawesson’s reagent.
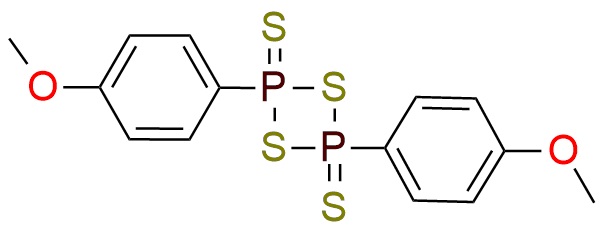
Synonyms: 2,4-bis(4-methoxyphenyl)-1,3,2,4- dithiadiphosphetane 2,4-disulfide; 2,4-bis(4-methoxyphenyl)-2,4-dithioxo-1,3,2,4-dithiadiphosphetane; 2,4-bis-(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane 2,4-disulfide; 4-methoxyphenylthiophosphoric cyclic di(thioanhydride); LR
Selected publication
-
2,4-Bis(4-Methoxyphenyl)-1,3,2,4-Dithiadiphosphetane 2,4-Disulfide.
Voss J.; Voss J. Encyclopedia of Reagents for Organic Synthesis 2006. DOI: 10.1002/047084289X.rb170.pub2