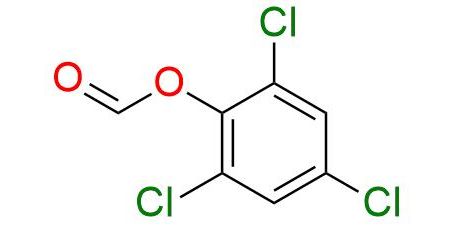
CAS 4525-65-9, Cat. No EN300-7411013
Reagent the CO precursor
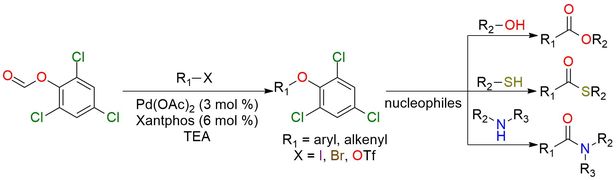
2,4,6-Trichlorophenyl formate is an accessible crystalline CO precursor that proceeds to generate CO in the presence of NEt3. The reagent is a pale-yellow crystalline, soluble well in THF and DMF1,2. Decarbonylation proceeds at room temperature, after which CO easily undergoes Pd-catalyzed carbonylation of aryl/alkenyl halides and triflates1-3. Compared to similar reagents, the high reactivity of precursor allows usage to near stoichiometric levels of formate for carbonylation. The obtained trichlorophenyl esters can be converted to carboxylic acid derivatives such as thioesters or amides with high yields1.
Synonyms: phenol, 2,4,6-trichloro-, formate (7CI, 8CI); 2,4,6-Trichlorophenyl formate
Selected publication
-
Pd‐Catalyzed External‐CO‐Free Carbonylation: Preparation of 2,4,6‐Trichlorophenyl 3,4‐Dihydronaphthalene‐2‐Carboxylate.
Konishi H.; Ueda T.; Manabe K.; Ciesielski J.; Carreira E. Organic Syntheses 2014, 39–51. DOI: 10.1002/0471264229.os091.04
-
Trichlorophenyl Formate: Highly Reactive and Easily Accessible Crystalline CO Surrogate for Palladium-Catalyzed Carbonylation of Aryl/Alkenyl Halides and Triflates.
Ueda T.; Konishi H.; Manabe K. Org Lett 2012, 14 (20), 5370–5373. DOI: 10.1021/ol302593z
-
First Example of a Continuous-Flow Carbonylation Reaction Using Aryl Formates as CO Precursors.
Alonso N.; Juan de M. M.; Egle B.; Vrijdag J. L.; De Borggraeve W. M. et al.J Flow Chem 2014, 4 (3), 105–109. DOI: 10.1556/JFC-D-14-00005