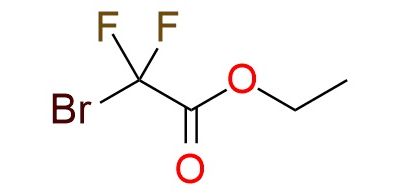
CAS 667-27-6, Cat. No EN300-39190
Reagent for difluoroalkylation
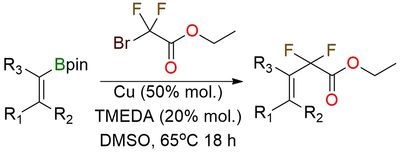
Ethyl bromodifluoroacetate is a versatile electrophilic reagent used for difluoroalkylation in organic synthesis1. It enables copper-mediated C–C coupling with alkenyl boronates, providing an efficient route to gem-difluorinated allyl acetates2. This reaction operates under mild conditions using copper powder and TMEDA, offering an alternative to traditional Suzuki couplings. The reagent is valuable for pharmaceutical and synthetic chemistry, producing CF2-functionalized building blocks with high efficiency. This moisture-sensitive liquid should be stored under an inert atmosphere to prevent hydrolysis. The method is scalable up to 76g, making it practical for large-scale applications.
Synonyms: ethyl 2-bromo-2,2-difluoroacetate; ethylbromodifluoroacetate; bromodifluoroacetic acid ethyl ester; ethyl difluorobromoacetate; acetic acid, bromodifluoro-, ethyl ester; 2-bromo-2,2-difluoroacetic acid ethyl ester; ethyl bromodifluoroacetate
Selected publications
-
Ethyl Bromodifluoroacetate.
Konas D. W.; Coward J. K.; Bos M. Encyclopedia of Reagents for Organic Synthesis 2018, 1–12. DOI: 10.1002/047084289X.rn00515.pub2
-
Copper-Mediated C−C Coupling of Alkenyl Boronates and Bromodifluoroacetates.
Yurov Y.; Laniush K.; Hryschuk O. V.; Liashuk O. S.; Grygorenko O. O. Adv Synth Catal 2025. DOI: 10.1002/adsc.202401489