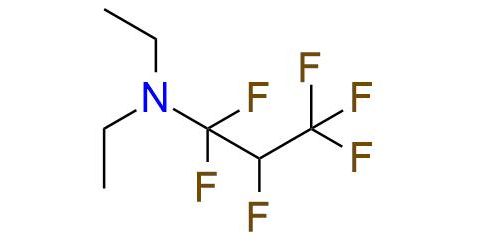
CAS 309-88-6, Cat. No EN300-118467
Deoxofluorinating reagent
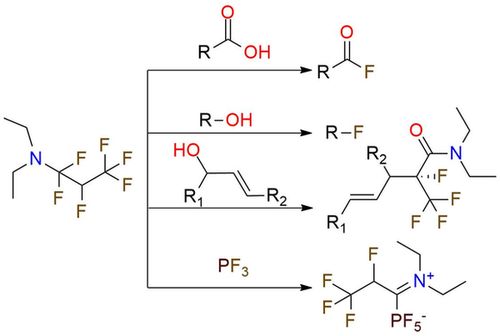
Ishikawa's reagent, as an improvement on Yarovenko's reagent, is a shelf-stable white powder that has proven to be a valuable addition to the arsenal of chemists working in the field of fluorination. It allows the soft conversion of alcohols into alkyl fluorides, carboxylic acids into acyl fluorides, and the preparation of 2,3,3,3-tetrafluoropropionate esters and other fluorine-containing carbonyl compounds1,2. Additionally, it serves as a key reagent in the synthesis of α-fluoro-α-(trifluoromethyl)-ɣ,σ-unsaturated amides from allylic alcohol3. Beyond fluorination capabilities, Ishikawa's reagent finds applications in the formation of iminium phosphate salts from phosphanes and phosphoranes4.
Synonyms: PPDA; N,N-diethyl-1,1,2,3,3,3-hexafluoropropylamine; hexafluoropropene diethylamine
Selected publication
-
Ishikawa’s Reagent – a Valuable Source for Fluoroorganic Iminium Salts.
Keßler M.; Neumann B.; Stammler H.; Hoge B. Z Anorg Allg Chem 2021, 647 (4), 225–230. DOI: 10.1002/zaac.202000198
-
Diethyl(1,1,2,3,3,3-Hexafluoropropyl)Amine.
Filler R.; Hofferberth J.; Beckett J. Encyclopedia of Reagents for Organic Synthesis 2007. DOI: 10.1002/9780470842898.rd196.pub2
-
Novel Reaction of Allylic Alcohols with a Hexafluoropropene-Diethylamine Adduct (PPDA) to Form α-Fluoro-α-(Trifluoromethyl)-γ,δ-Unsaturated Amides.
Ogu K.; Akazome M.; Ogura K. Tetrahedron Lett 1998, 39 (3–4), 305–308. DOI: 10.1016/S0040-4039(97)10548-2
-
F-Propene-Dialkylamine Reaction Products as Fluorinating Agents.
Takaoka A.; Iwakiri H.; Ishikawa N. Bull Chem Soc Jpn 1979, 52 (11), 3377–3380. DOI: 10.1246/bcsj.52.3377