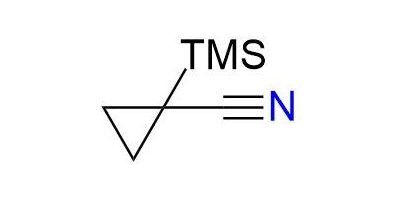
CAS 91686-39-4, Cat. No EN300-57100
Reagent for the introduction of cyclopropyl groups
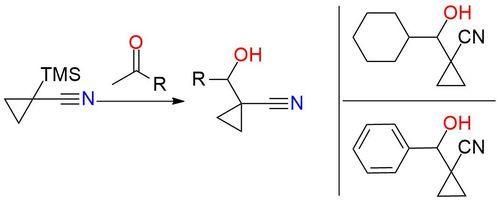
1-Trimethylsilylcyclopropane-1-carbonitrile is a stable, operationally simple procedure reagent for attaching cyclopropyl groups1. It is an air-stable white powder, solvable in THF, that under mild reaction conditions with TBAF or BTAF forms a cyclopropyl anion that can undergo aldol condensation reactions to form a C‑C bond. The reagent exhibits predictable positional selectivity and boasts a high level of compatibility with various functional groups2. This makes it an excellent option for polyfunctional substrates, enabling the construction of complex molecules and productive for pharmaceutically important targets.
Synonyms: 1-trimethylsilylcyclopropane-1-carbonitrile; cyclopropanecarbonitrile, 1-(trimethylsilyl)
Selected publication
-
Silicon in Organic Synthesis. 27. A Method for the Generation of Electronegatively Substituted Cyclopropyl Anions under Preparatively Useful Conditions. Aldol Condensation with Carbonyl Partners.
Paquette L.; Blankenship C.; Wells G. J Am Chem Soc 1984, 106 (21), 6442–6443. DOI: 10.1021/ja00333a068
-
Desilylative Condensation Reactions of Electronegatively Substituted 1-(Trimethylsilyl)Cyclopropanes.
Blankenship C.; Wells G.; Paquette L. Tetrahedron 1988, 44 (13), 4023–4032. DOI: 10.1016/S0040-4020(01)86653-9