Bioorthogonal chemical reactions are closely associated with the characteristics of “click” chemistry, occurring with high selectivity and fast reaction kinetics in vivo. Consequently, these reactions found use as multipurpose tools for chemical biology. The Inverse-electron-Demand Diels–Alder (iEDDA) reaction between tetrazines and strained alkenes is fairly new ligation reaction, which displays rates 3-7 orders of magnitude faster than many bioorthogonal reactions. High reaction rates, biocompatibility, together with the ability of tetrazines to quench fluorescence of some fluorophores, widely used for fluorescent labeling, and recover it after iEDDA reaction (Figure 1) make tetrazine derivatives unique and versatile tools for bioortogonal chemistry. Figure 2 is showcasing possible approach to modification of commonly used fluorophore as fluoresceine (A) with tetrazines and application of tetrazine derivatives in DNA encoded libraries technologies (DELT), as the core scaffolds (B).
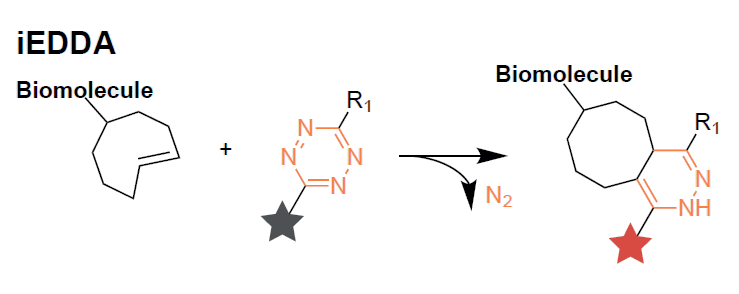 Figure 1. General scheme of IeDDA ligation.
Figure 1. General scheme of IeDDA ligation.
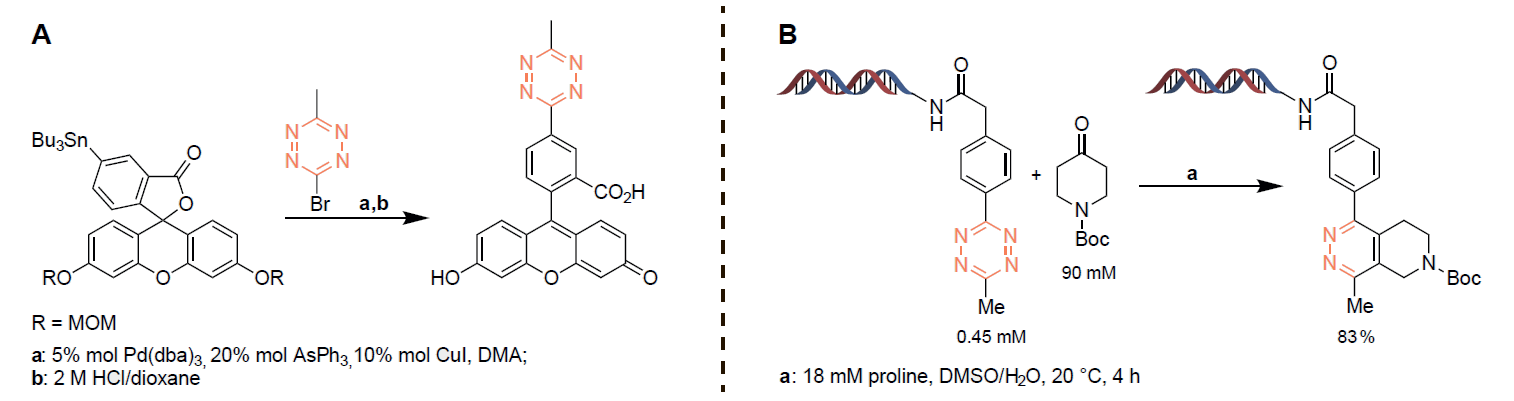 Figure 2. Tetrazine ligation of fluoresceine (A) and use of tetrazine core in DELT-compatible reaction (B).
Figure 2. Tetrazine ligation of fluoresceine (A) and use of tetrazine core in DELT-compatible reaction (B).
Download SD file
Download PDF file
We offer:
Currently, we have synthesized 8 tetrazine-containing building blocks, that are available in our store on a gram scale.
Pre-order:
We also have designed a library of tetrazine-containing building blocks. These molecules can be synthesized upon request.





























