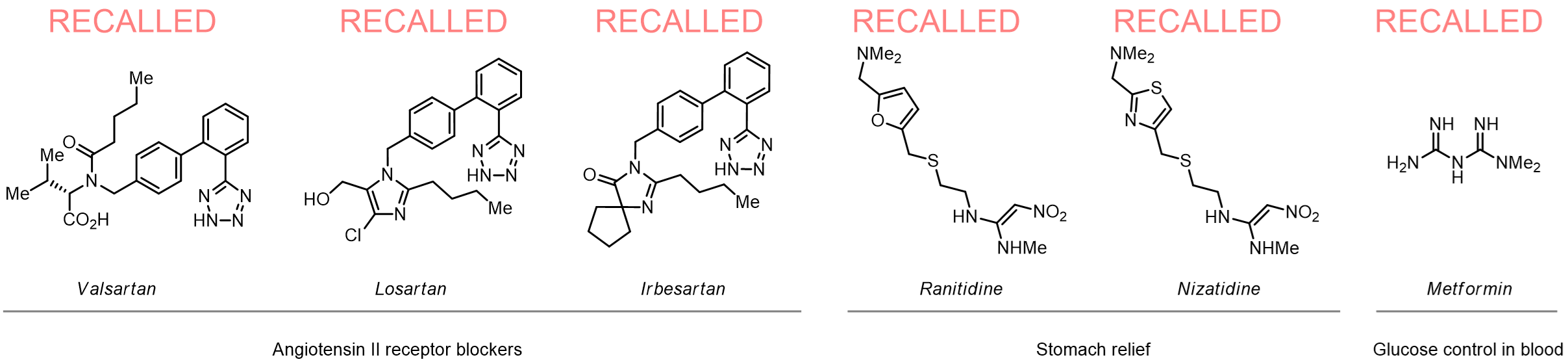
Since 2018, several drugs have been recalled from the market following the discovery of N-nitrosamine impurities in their commercial batches. The N-nitrosamines have been linked to an elevated risk of several cancers especially in case of continuous intake. Since 2020, the FDA and other authorities worldwide have mandated control of N-nitrosamine levels in commercial drugs.
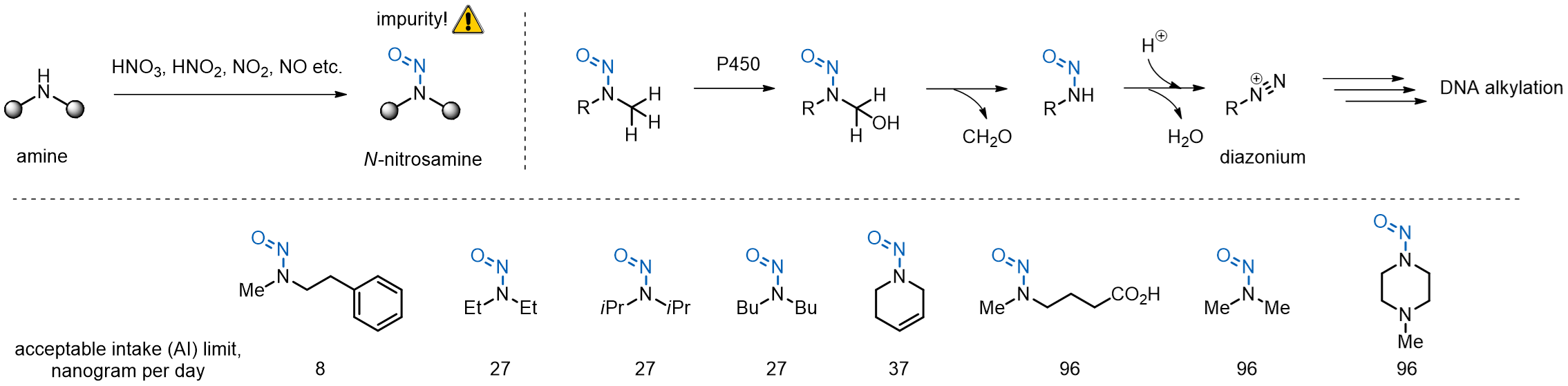
Mechanism
N-Nitrosamines can form as impurities in the process of chemical drug production. Following the drug administration, oxidation of the N-nitrosamine species by cytochrome P450 leads to downstream production of cancerogenic diazonium salts that damage DNA by alkylation of susceptible nucleobases. Acceptable daily intake limits have been mandated by authorities for a large panel of N-nitrosamine species, which is, however, not explicit.
Download SD file
Download PDF file
We offer
More than 200 N-nitrosamines from stock on a 5-10 g scale.






































