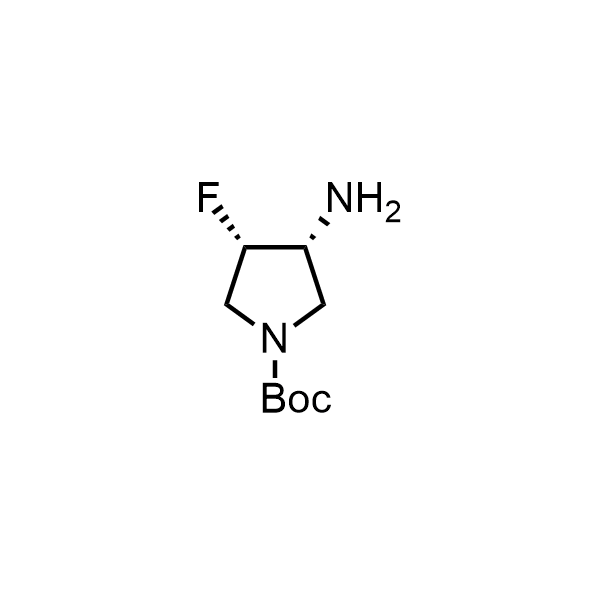
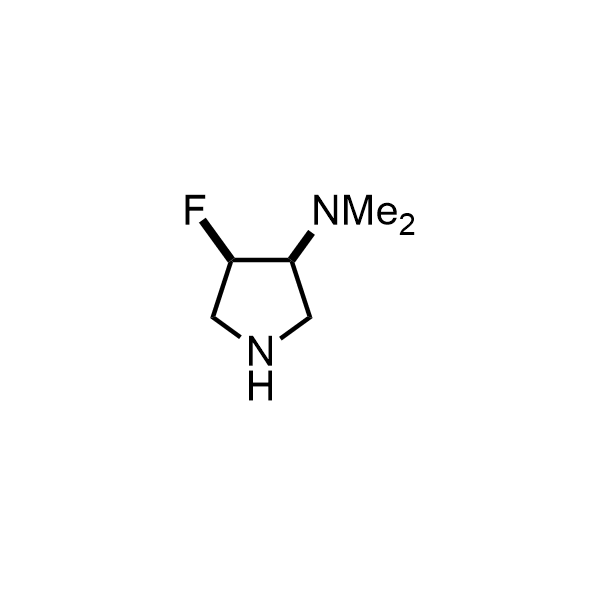
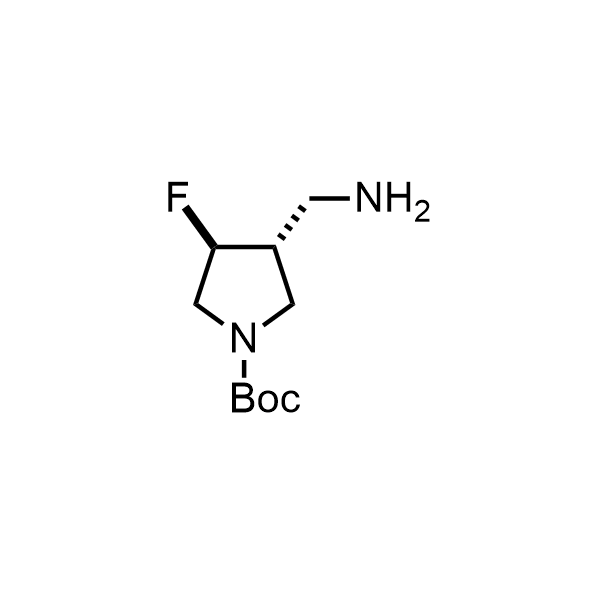
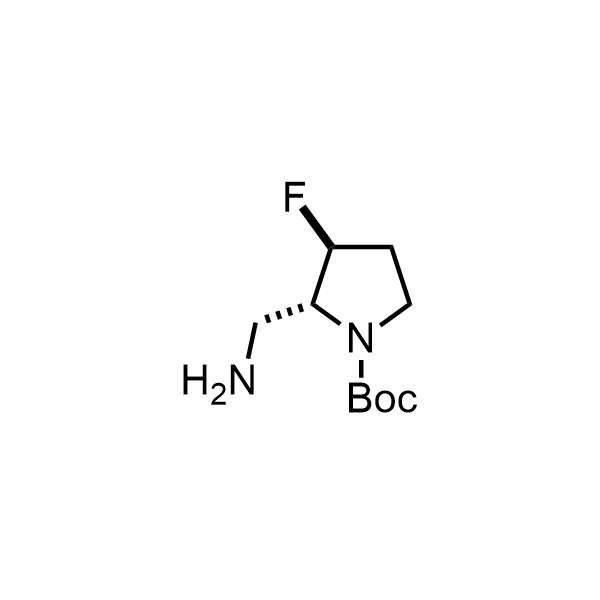
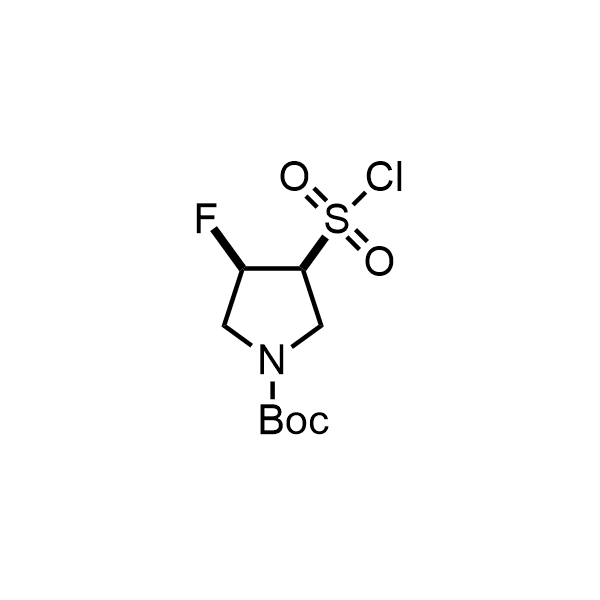
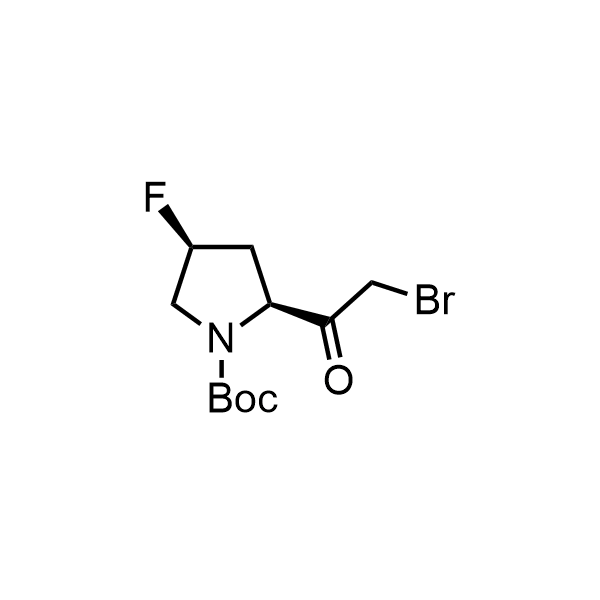
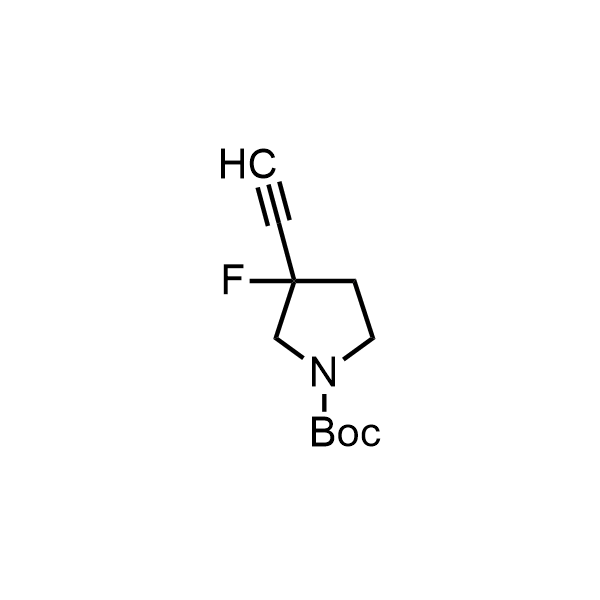
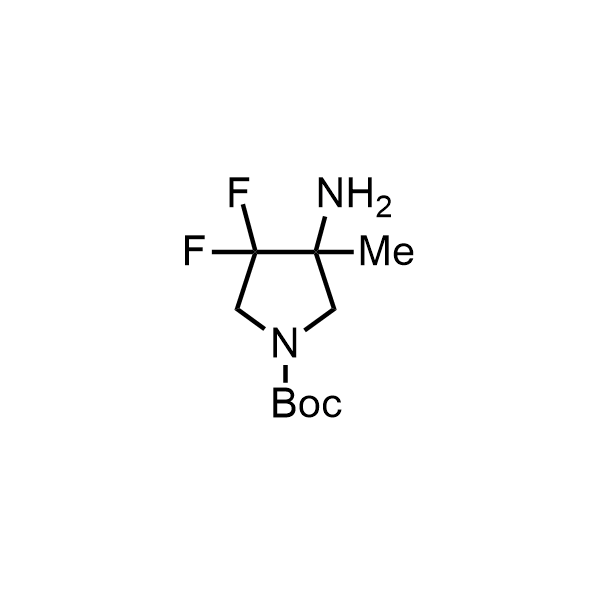
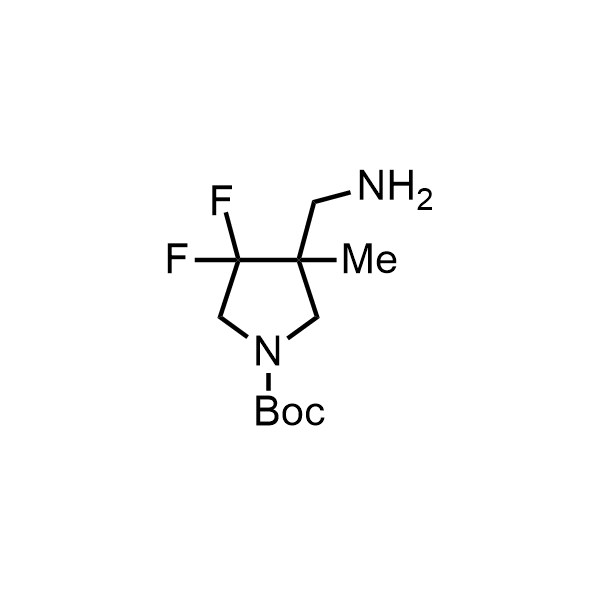
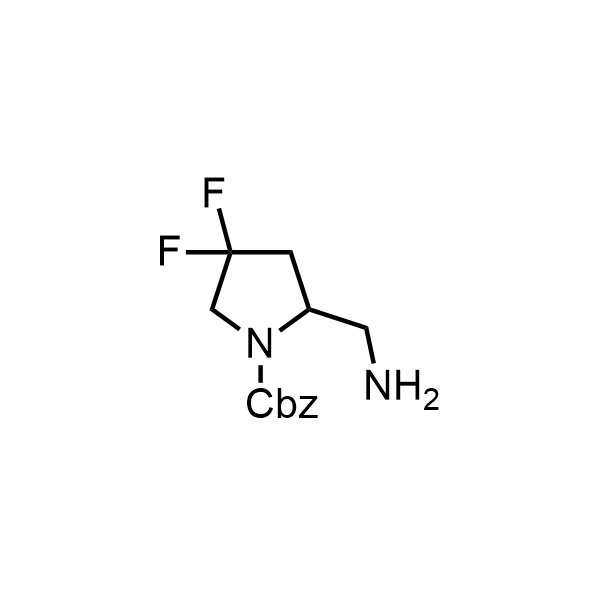
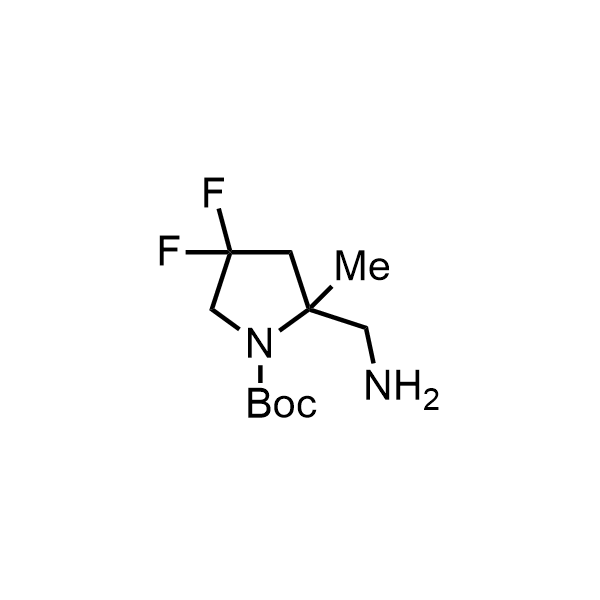
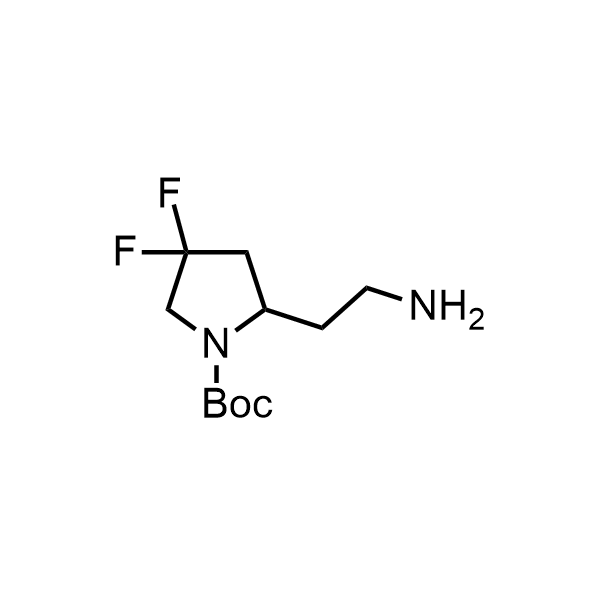
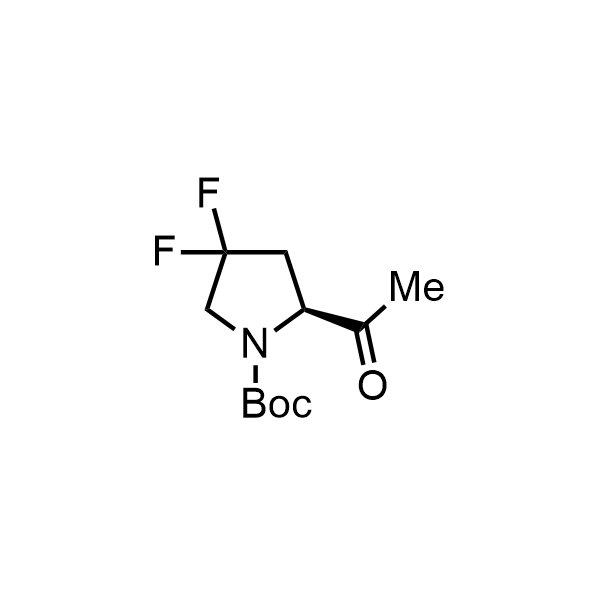
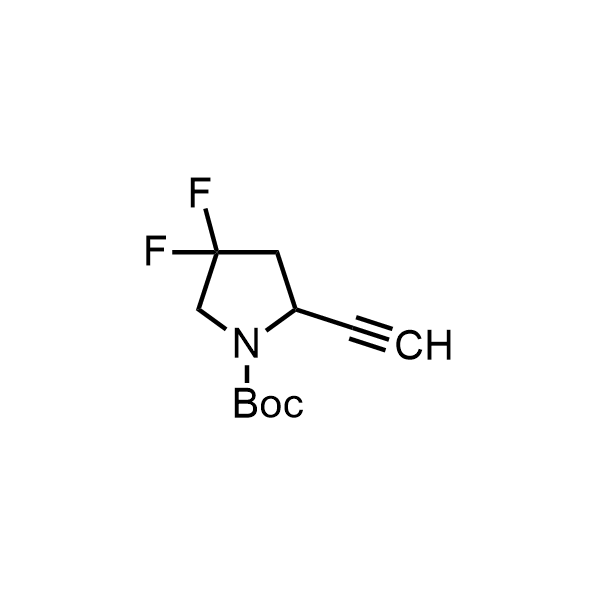
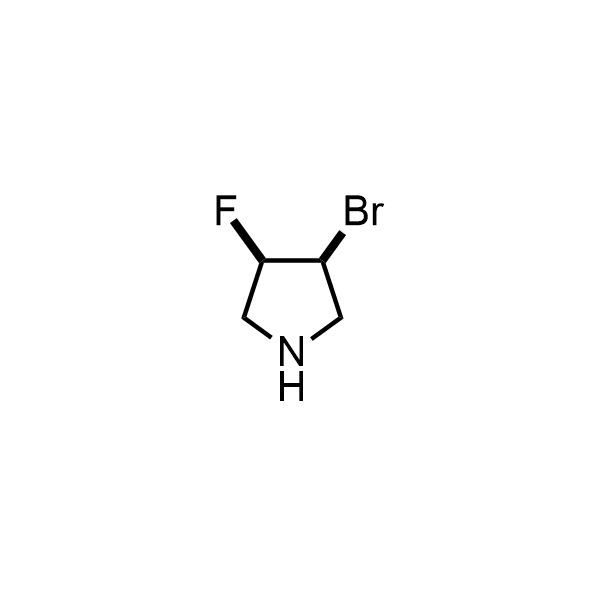
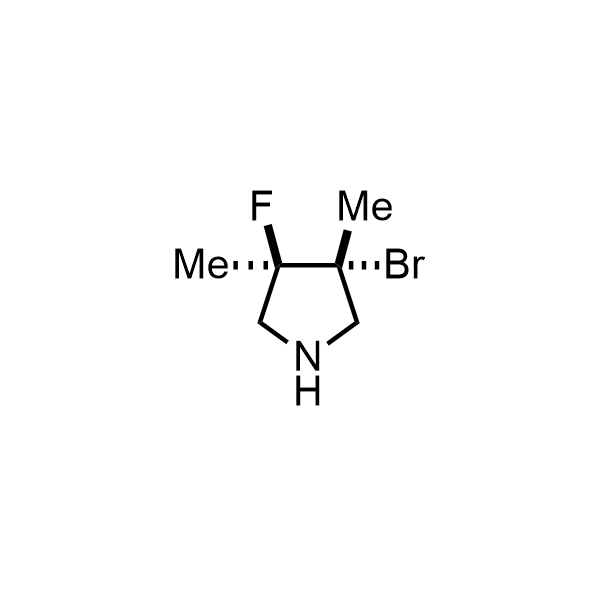
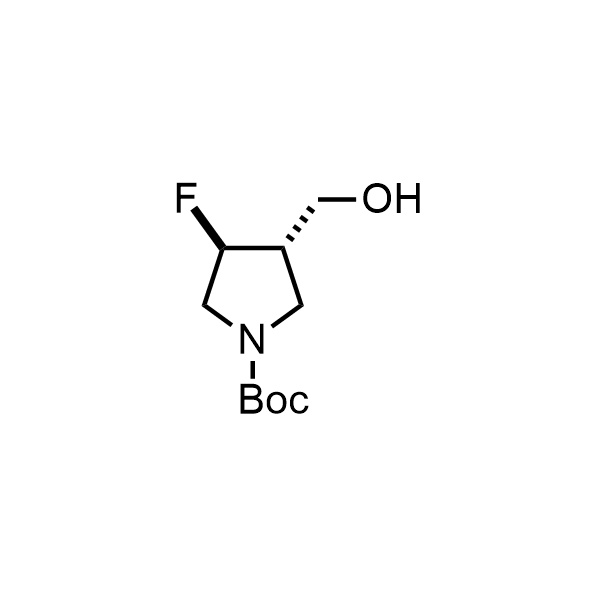
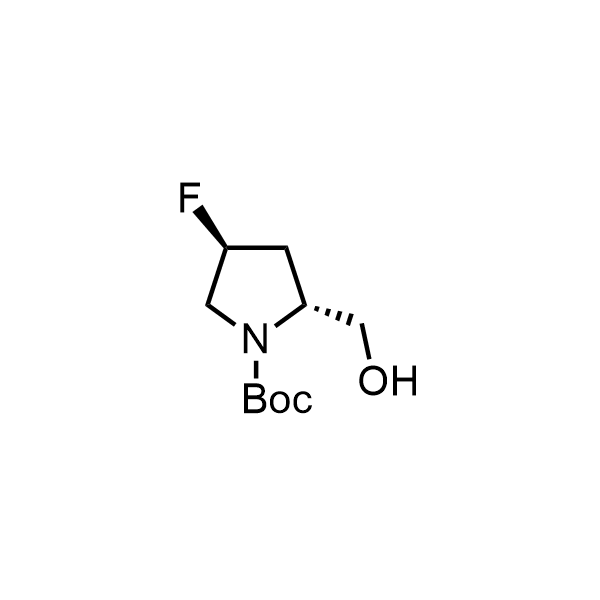
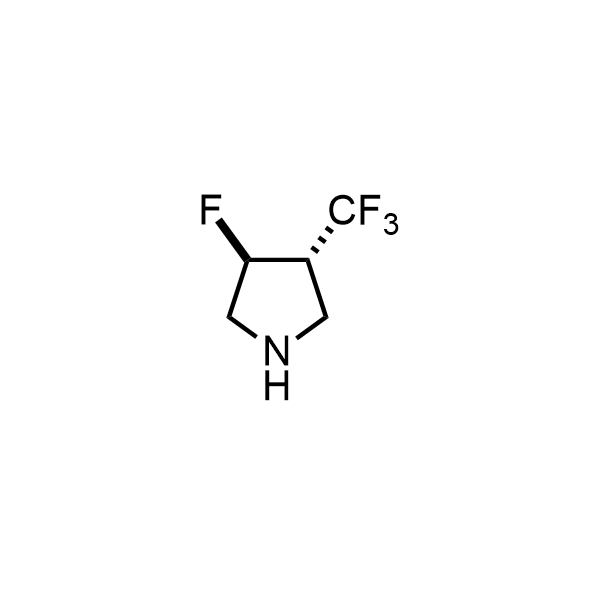
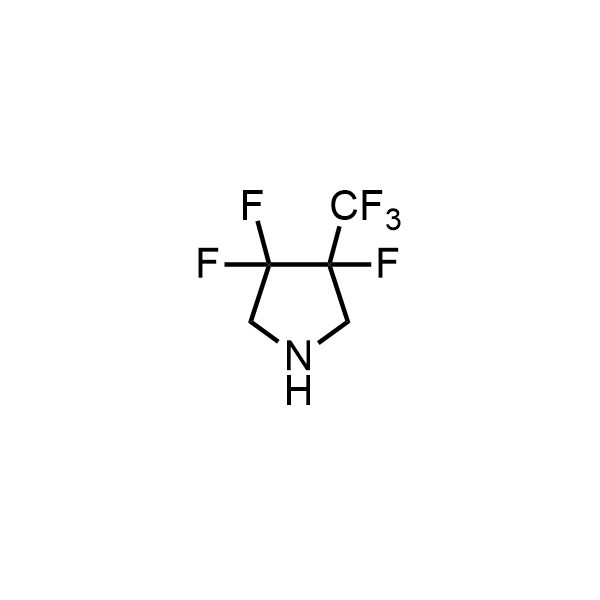
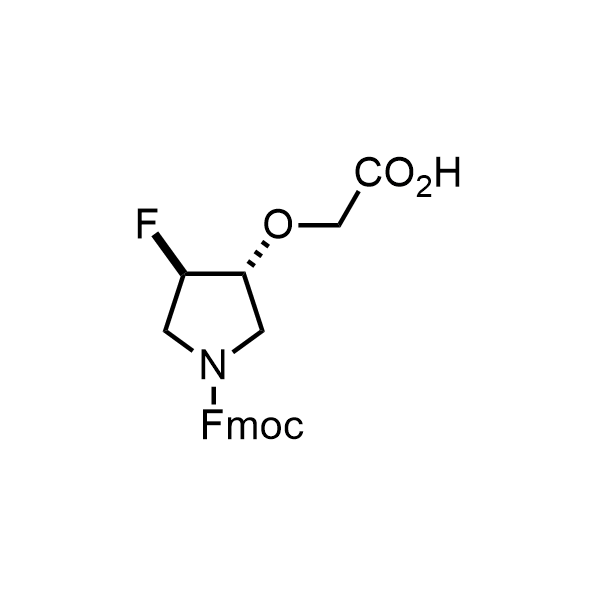
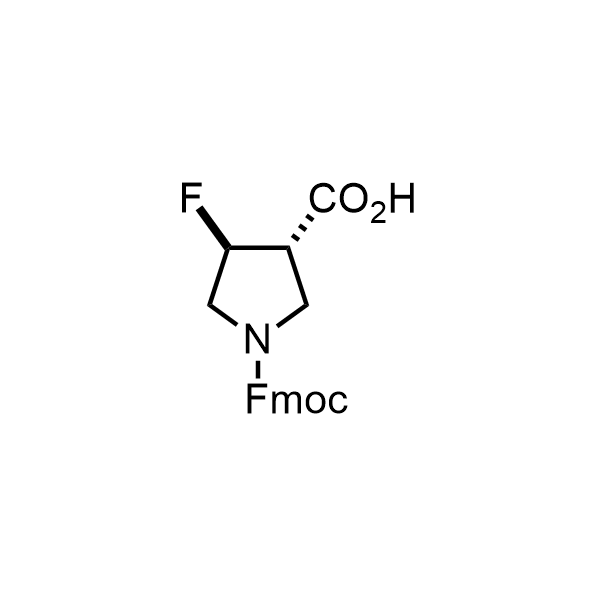
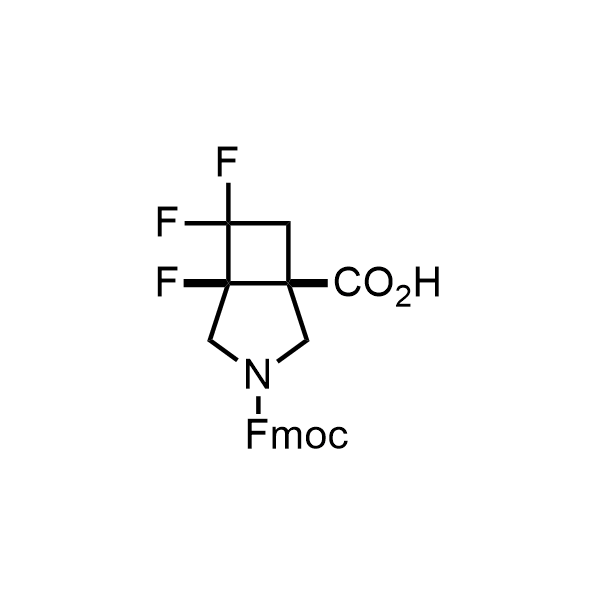
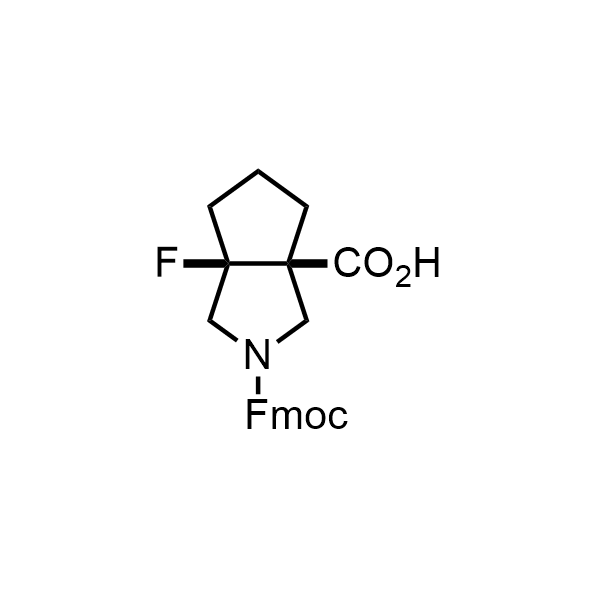
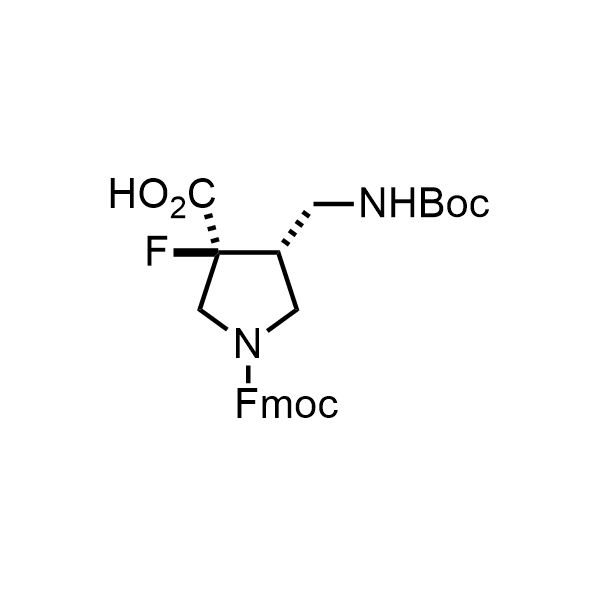
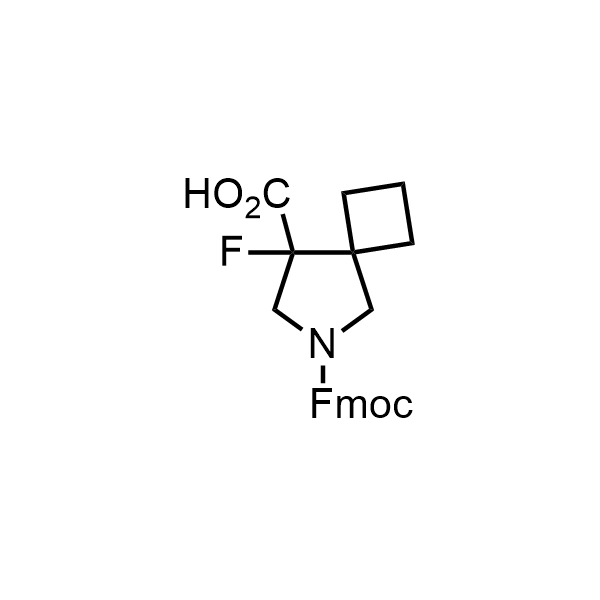
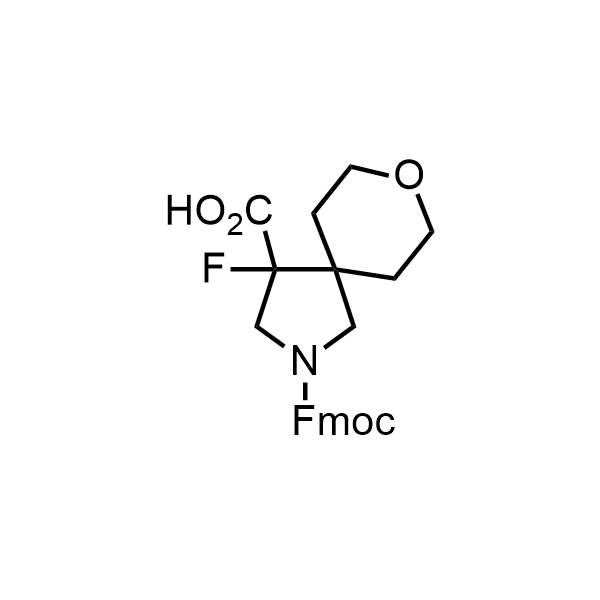
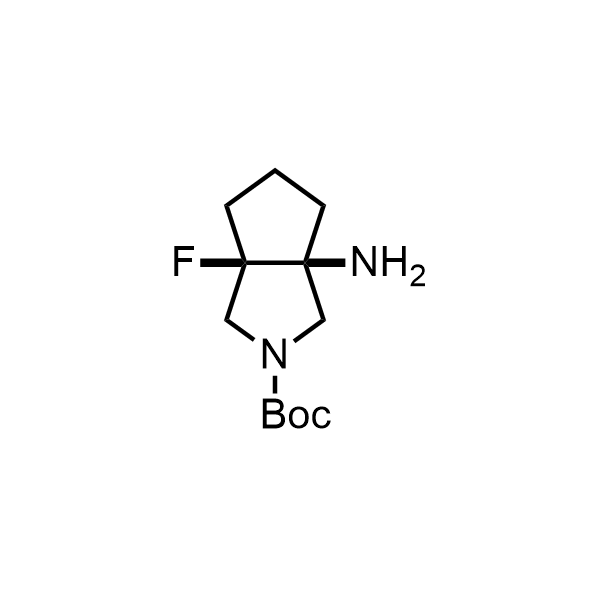
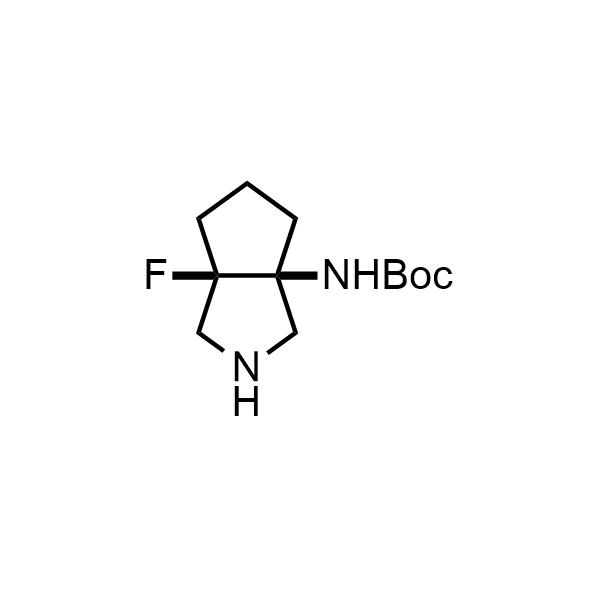
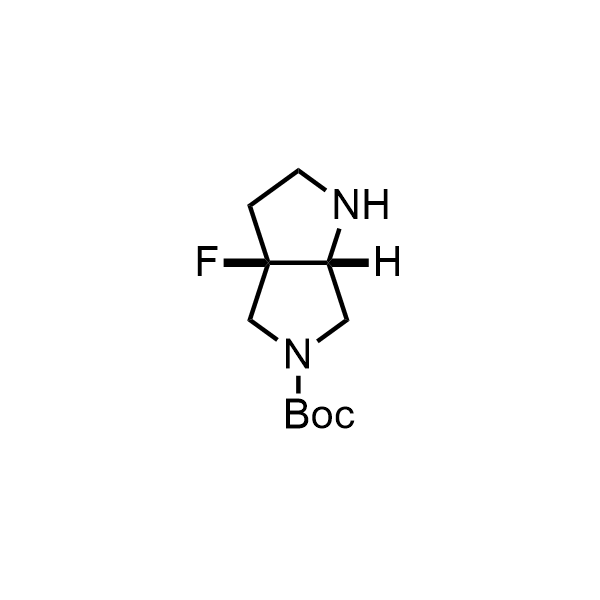
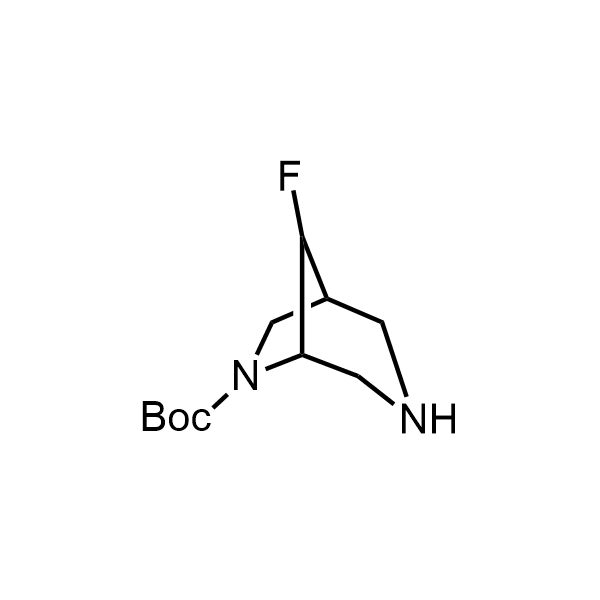
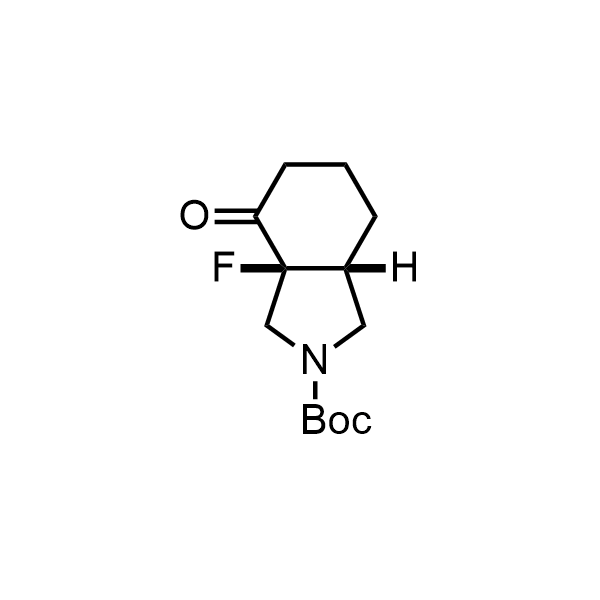
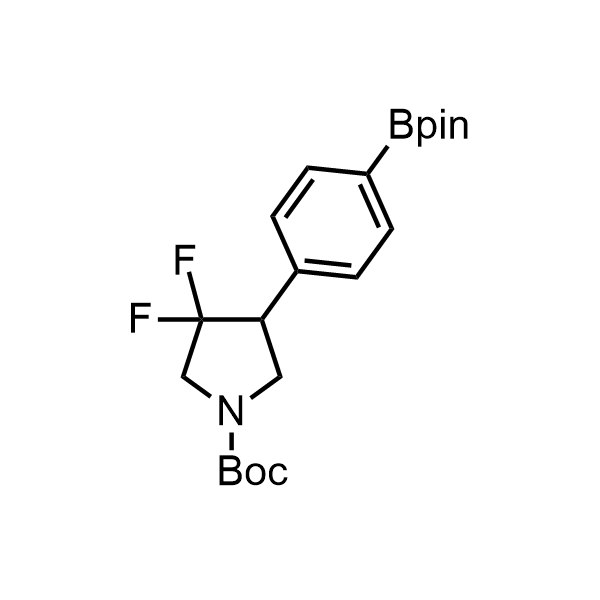
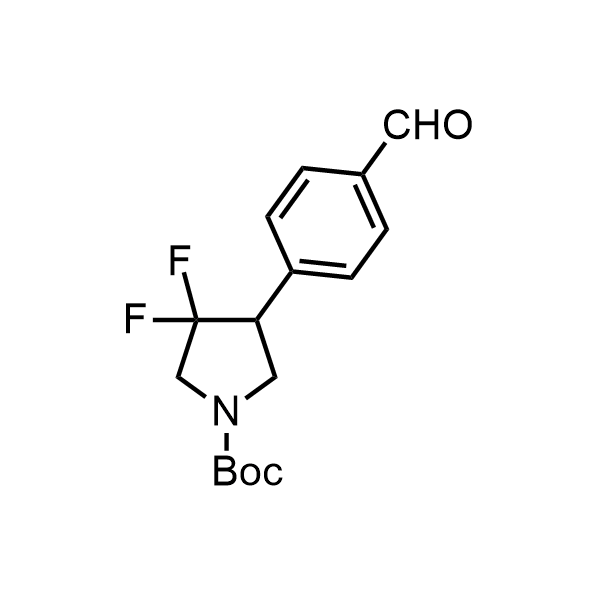
Pyrrolidine ring is the third most common heterocycle in the newly approved drug structures. However, the basicity of the nitrogen atom poses serious problems when protonation prevents passive permeability. Fluorination lowers the pKa to near or below physiological values, thus creating an optimal balance of the activity and permeability. The electronic effect of fluorine also enhances the dynamics of the amide and related conjugated structures. Finally, fluorination helps block major metabolic sites thus creating better bioavailable molecules. Explore our rich collection of fluorinated pyrrolidine building blocks.
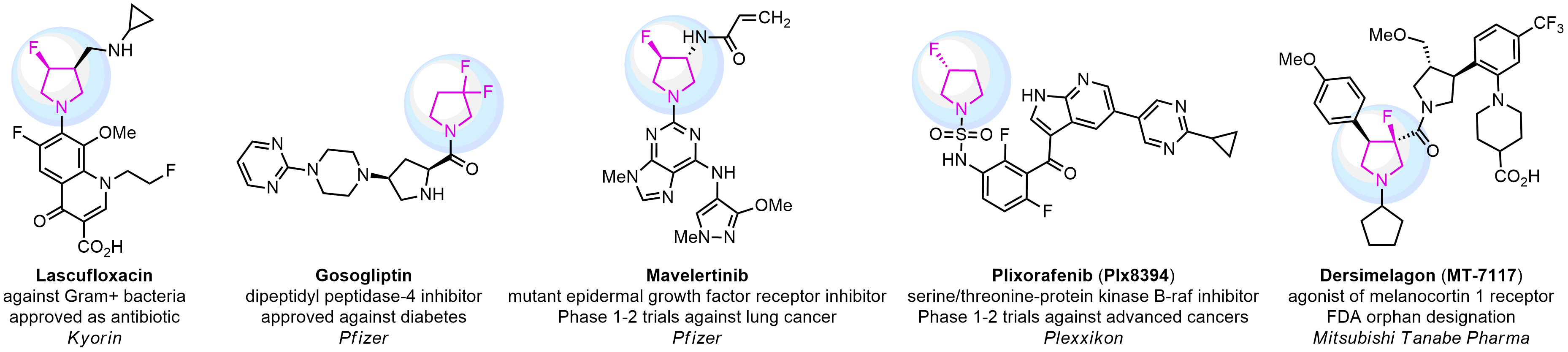
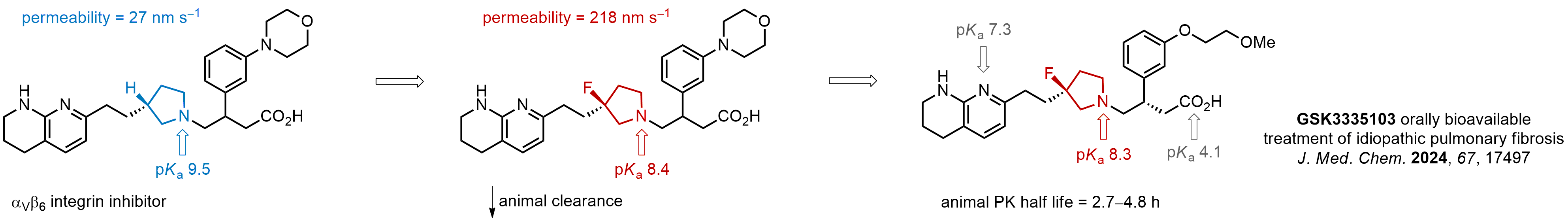
Case study
Download SD file
Download PDF file
We offer
Over 100 fluoropyrrolidines from stock on 5-10 gram scale.