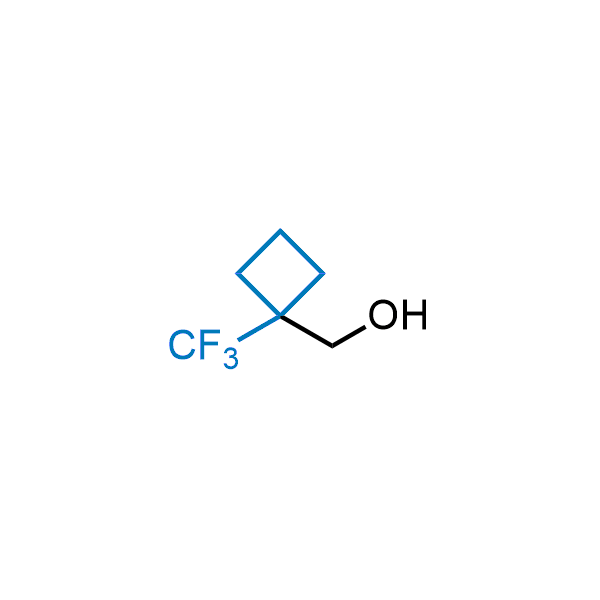
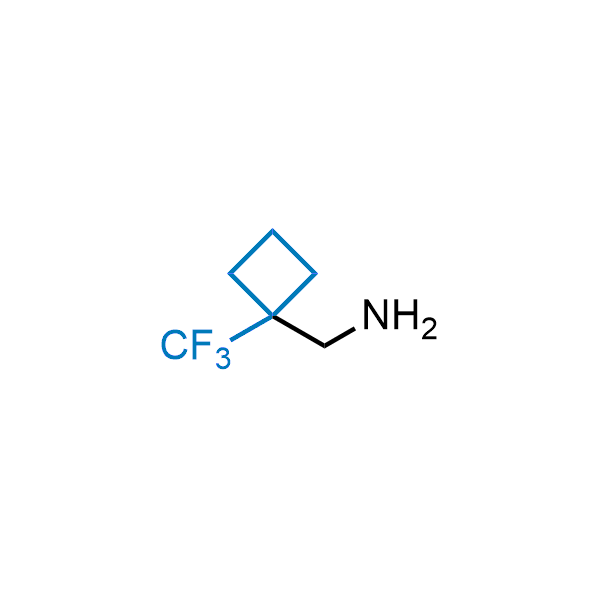
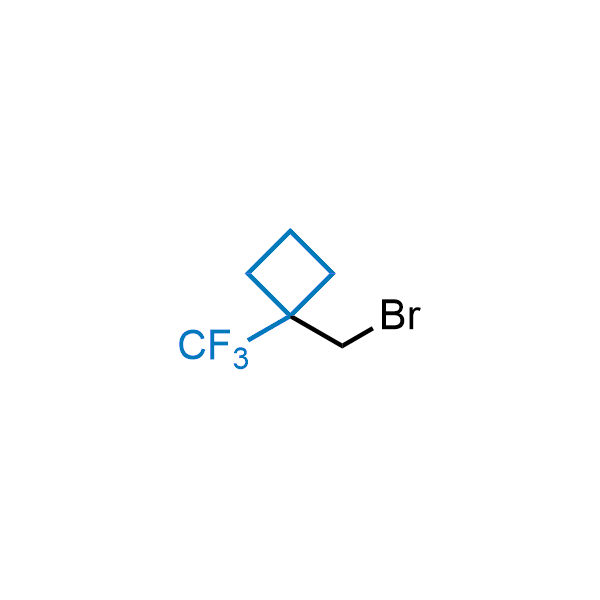
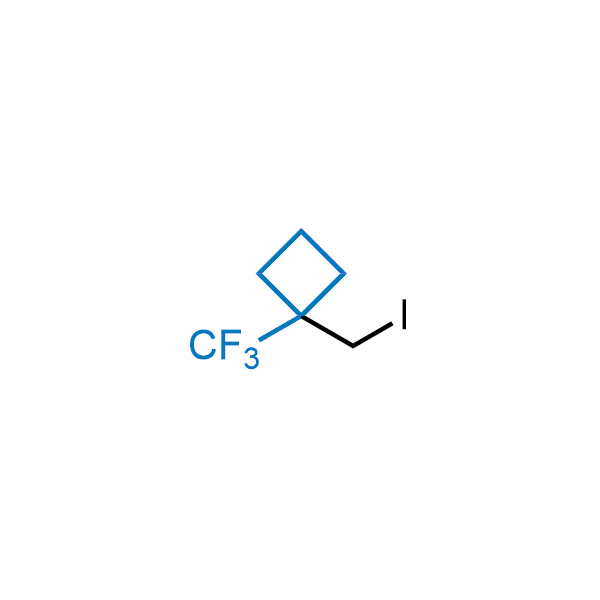
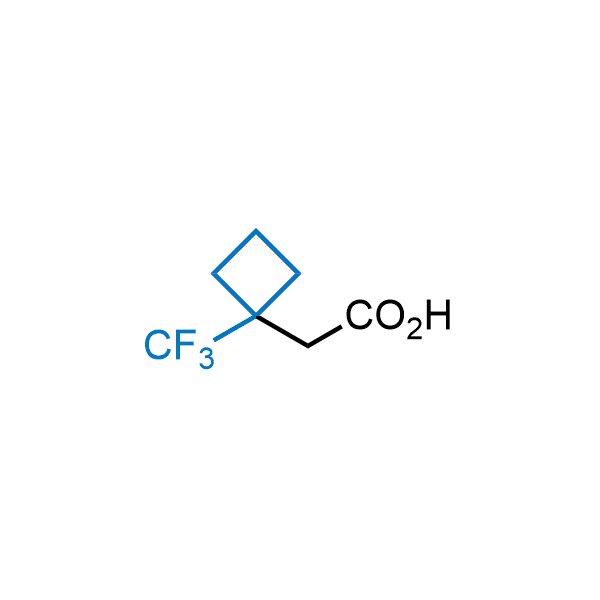
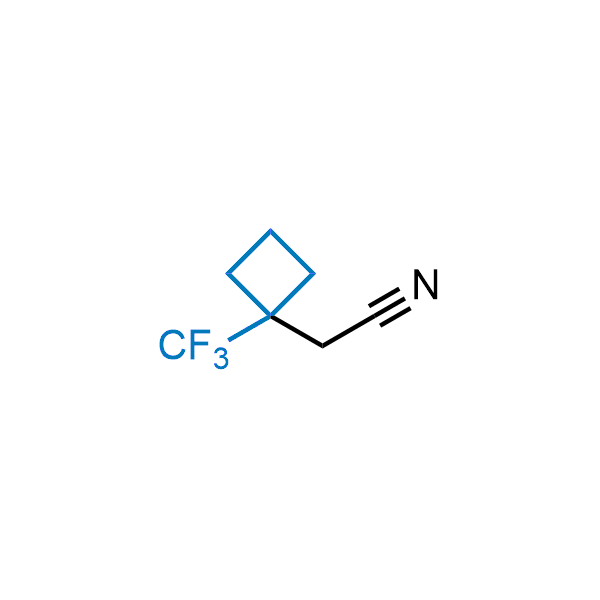
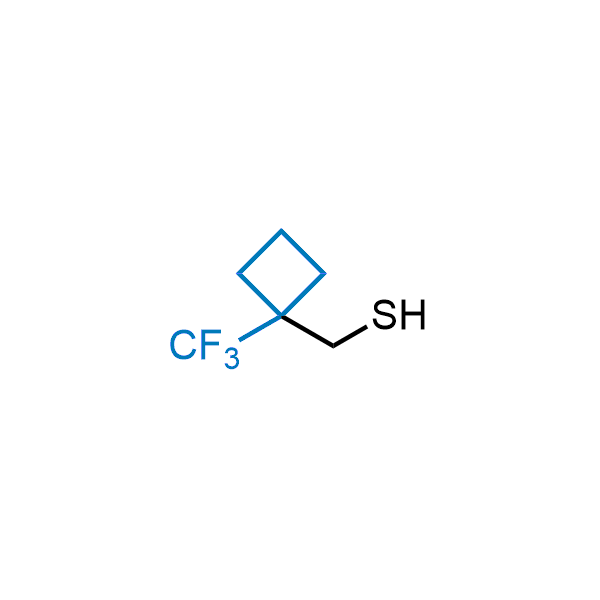
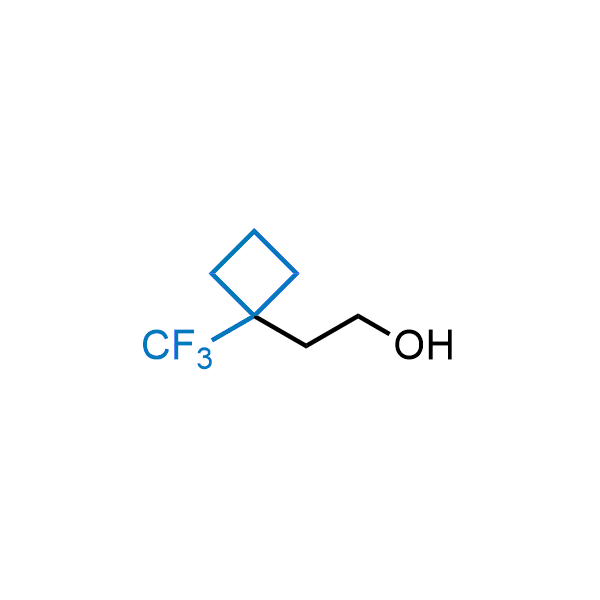
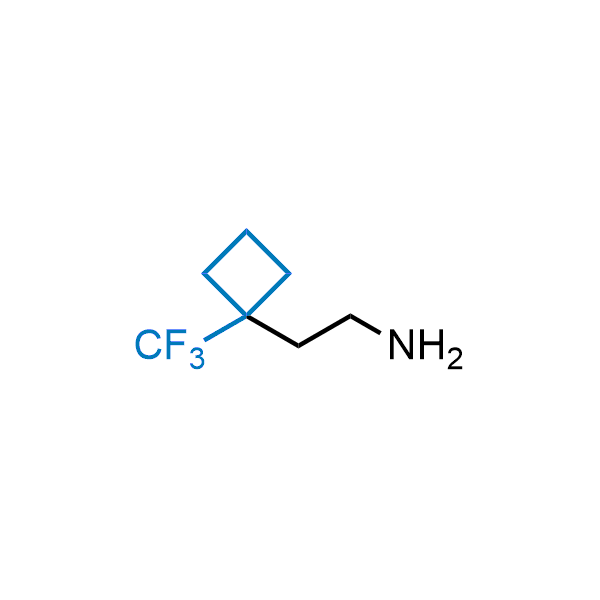
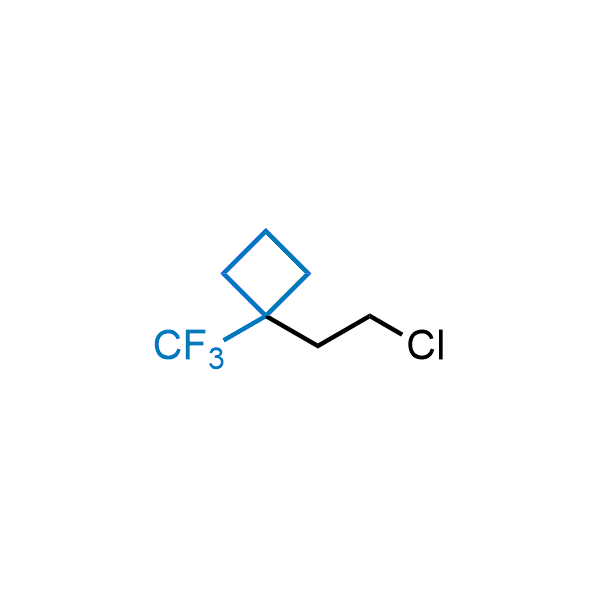
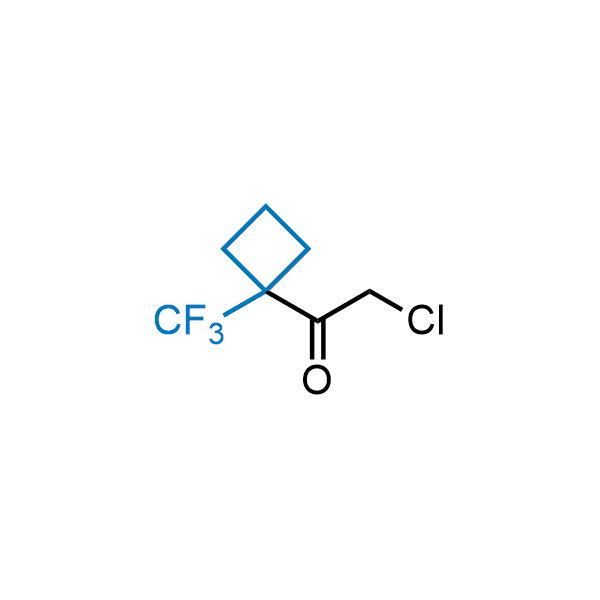
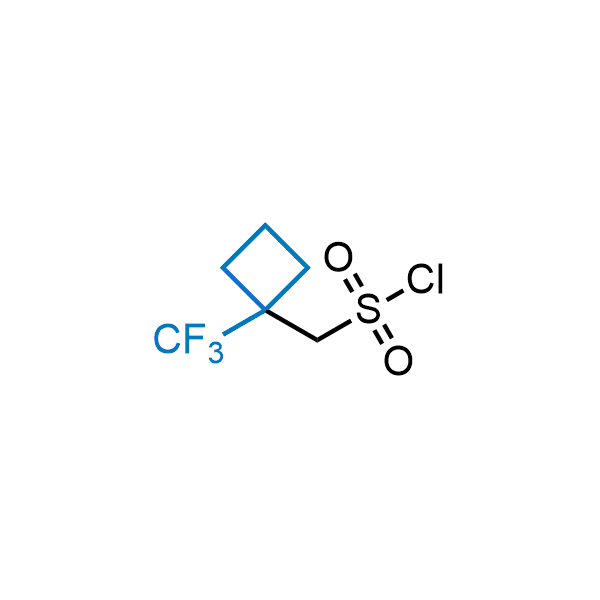
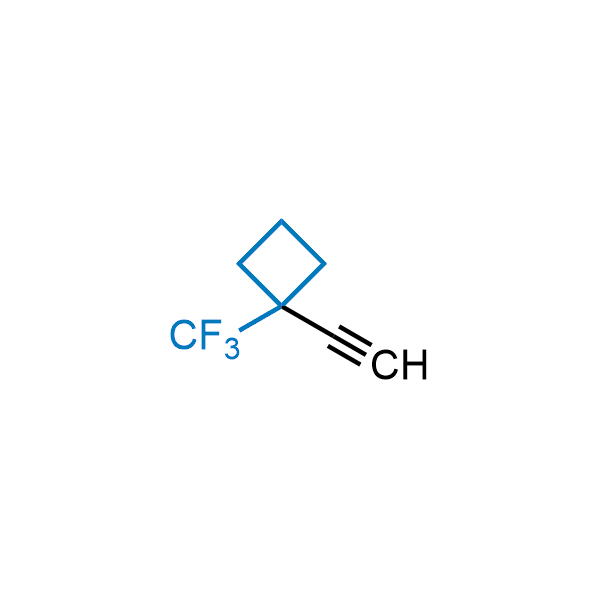
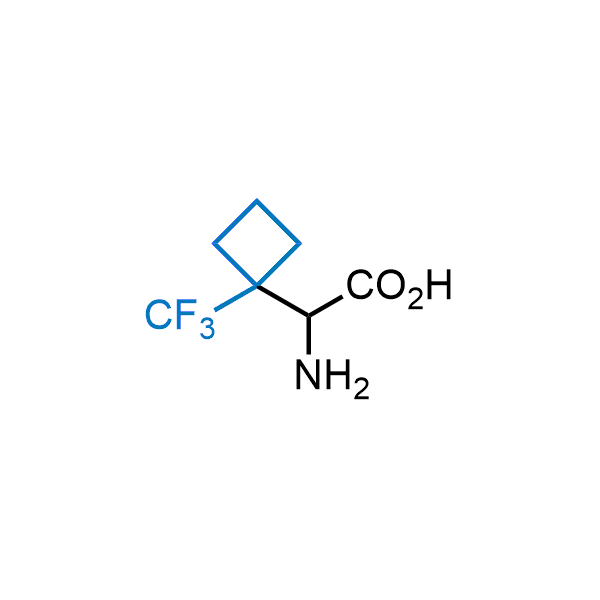
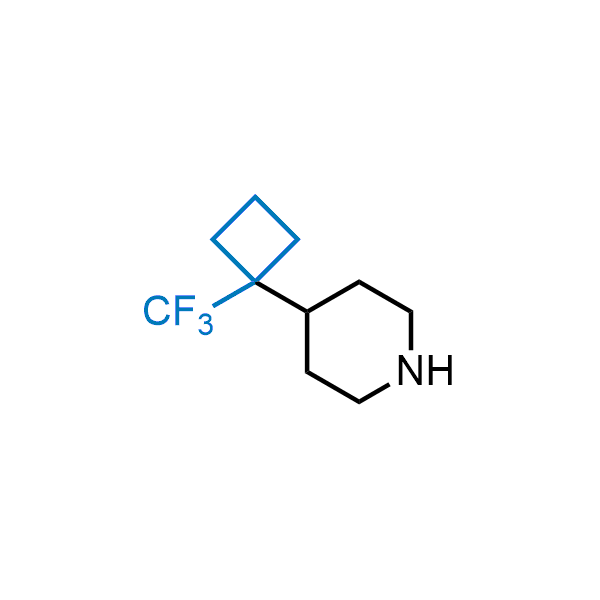
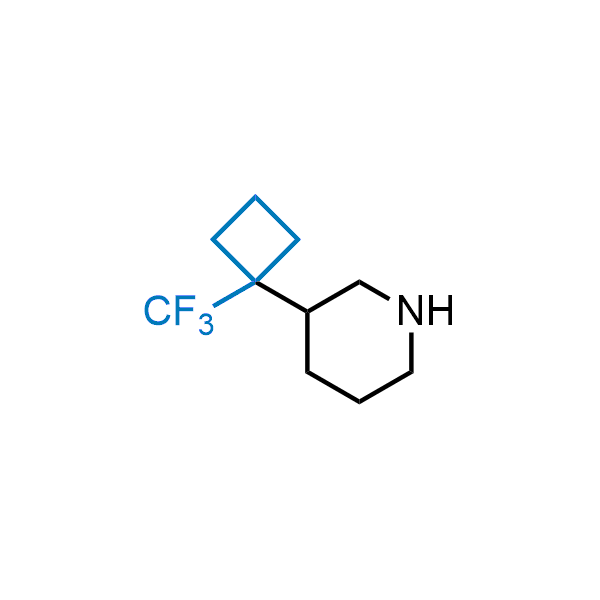
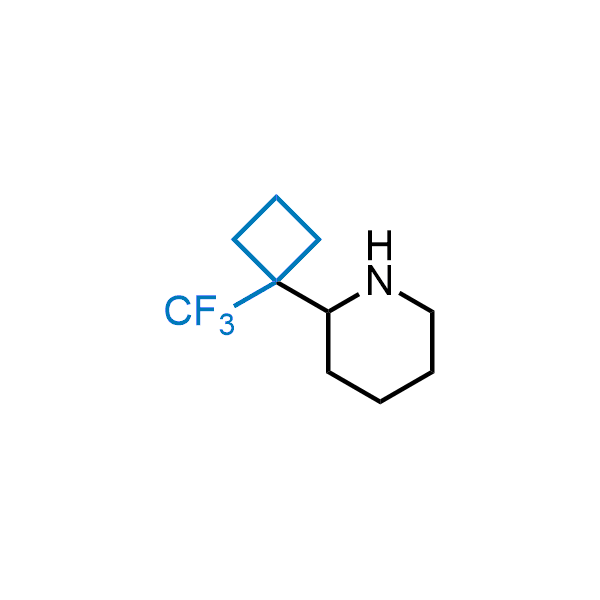
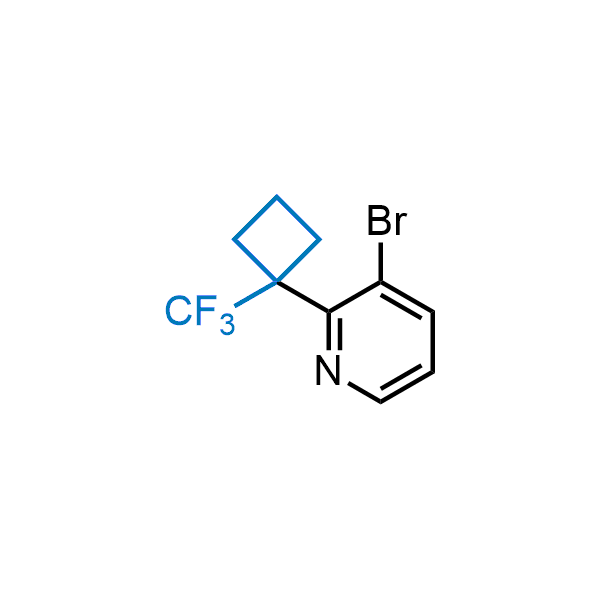
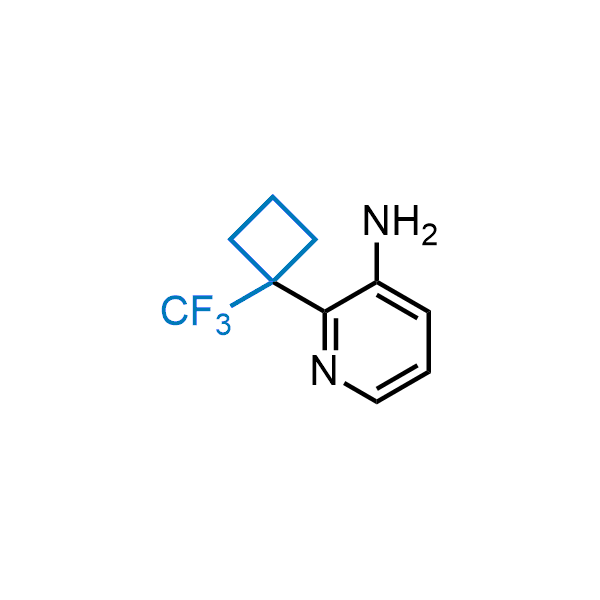
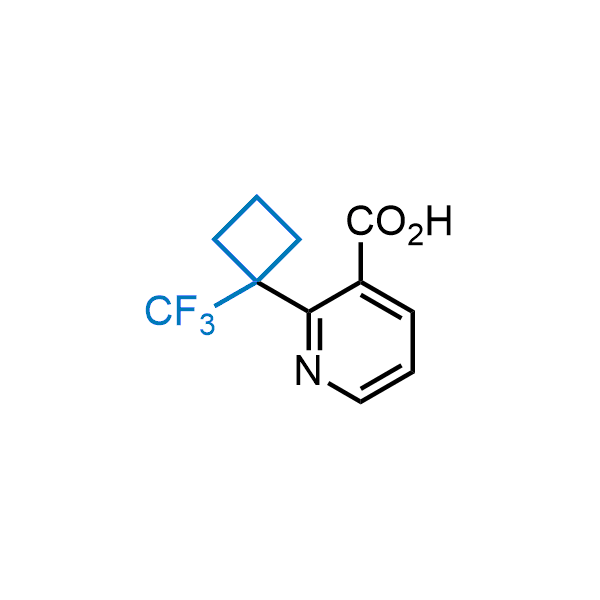
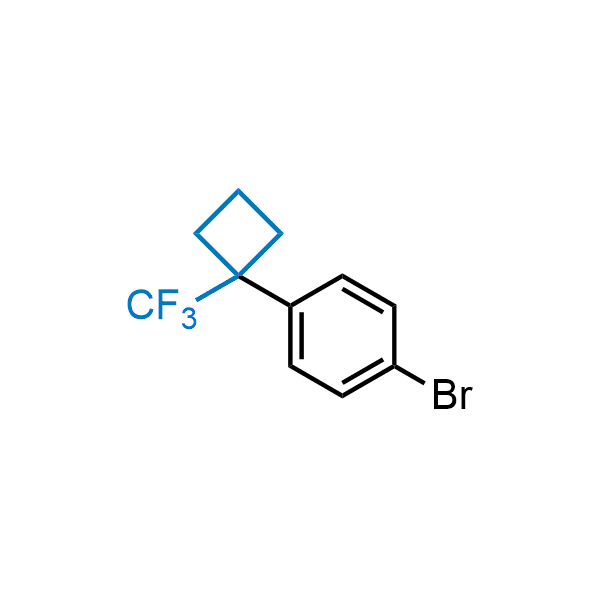
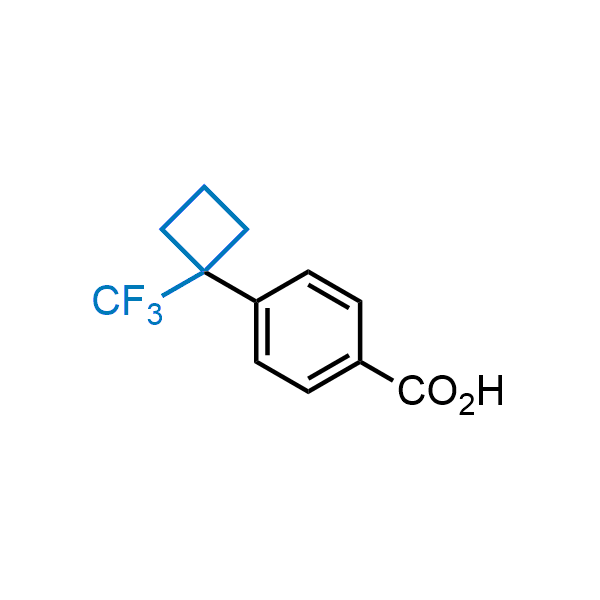
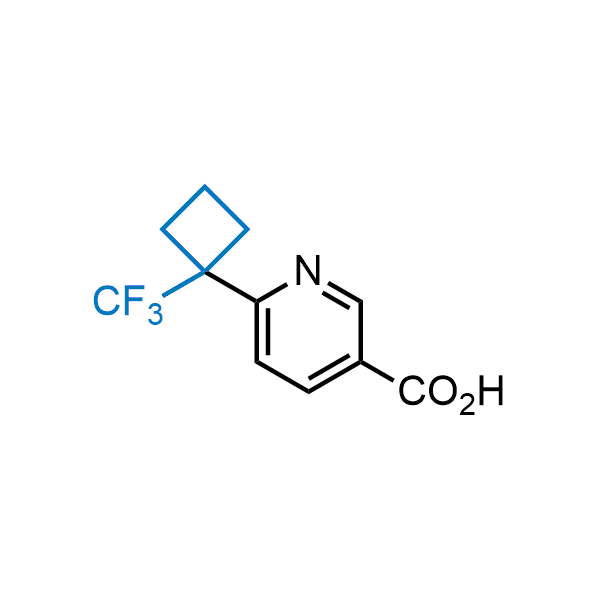
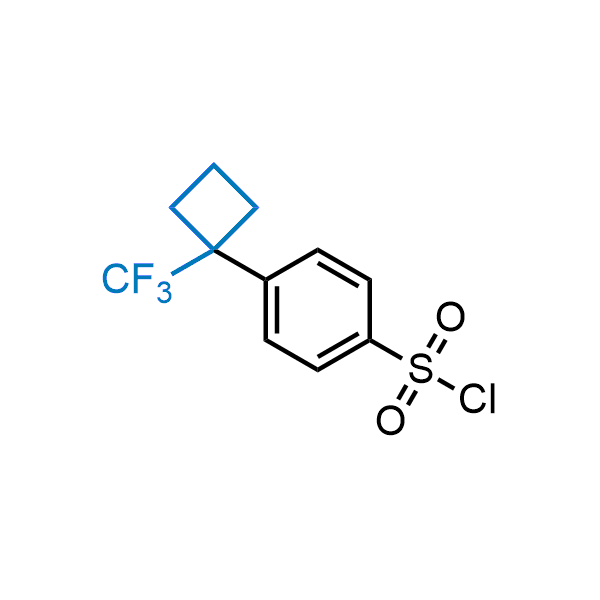
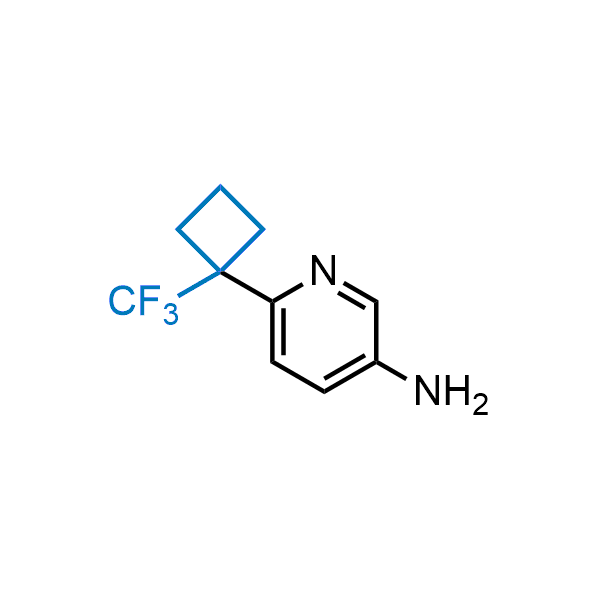
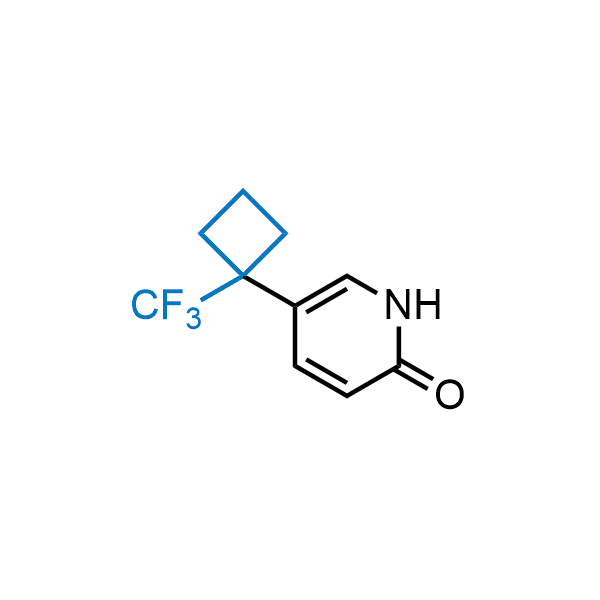
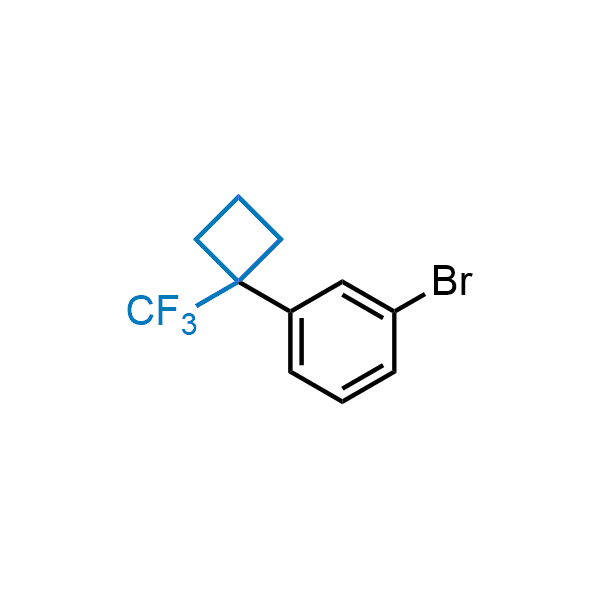
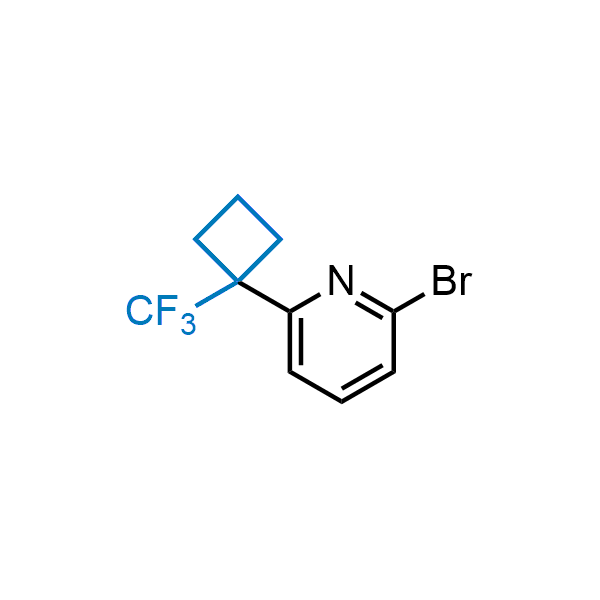
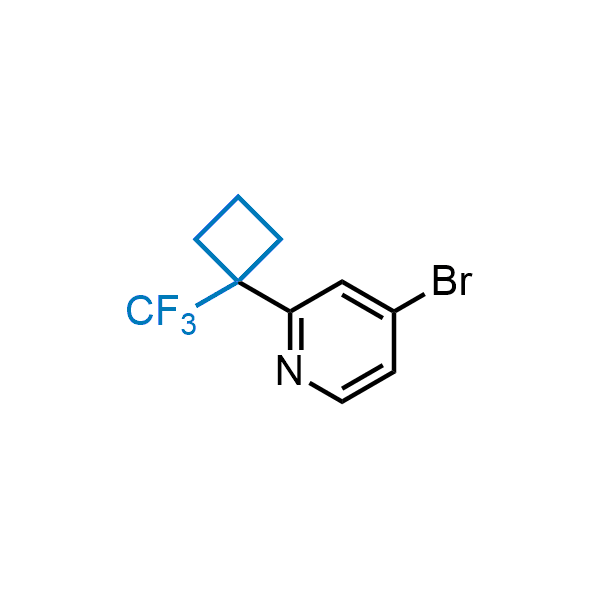
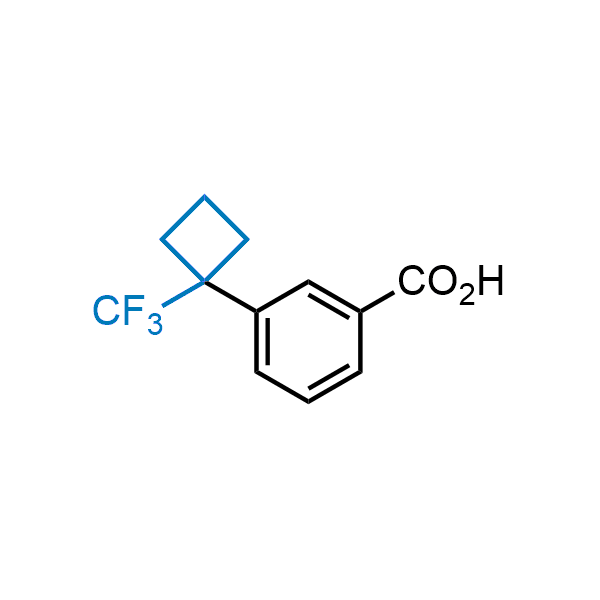
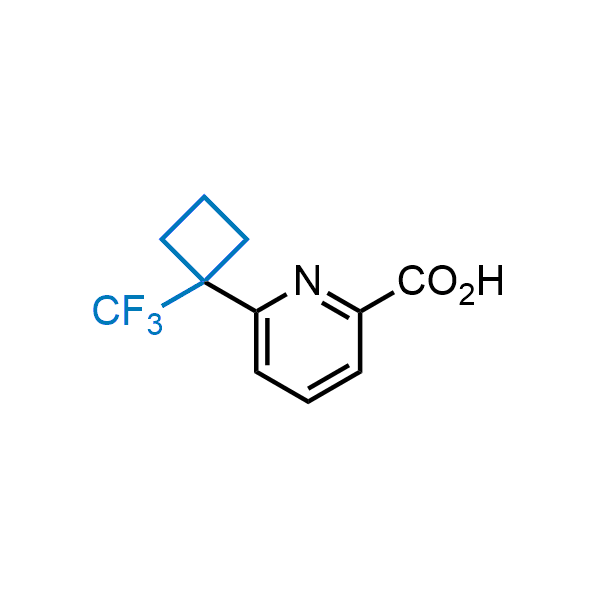
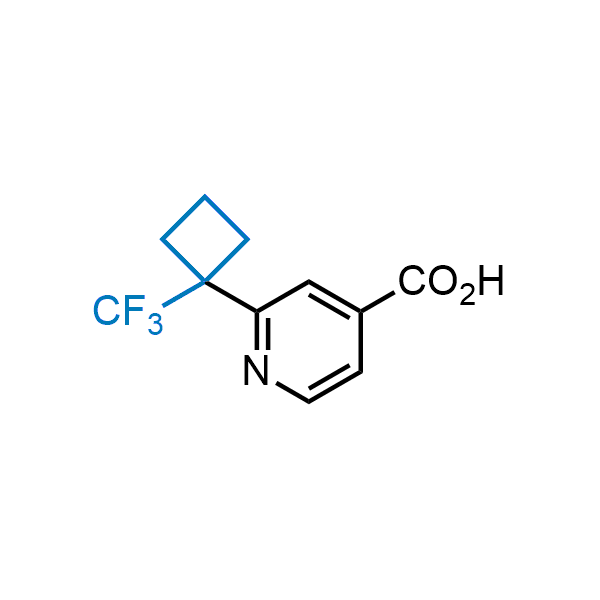
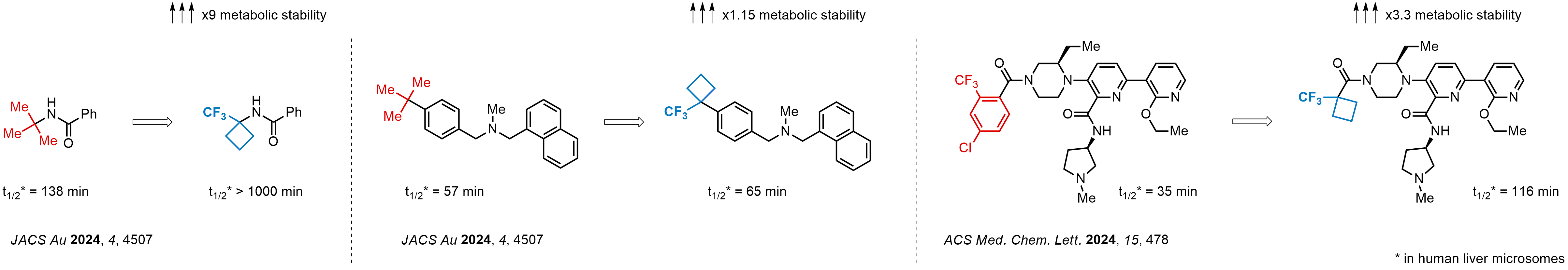
Achieving resistance to metabolic clearance is a key objective in optimizing the structures of orally available drugs. In pursuit of this goal, replacing tert-butyl groups aromatic moieties, and several other hydrophobic functional groups with a 1-trifluoromethyl-cyclobutyl group has shown promising results, maintaining bioactivity while reducing metabolic degradation. Recently, scientists at Enamine developed a practical synthetic approach to prepare dozens of building blocks featuring the 1-trifluoromethyl-cyclobutyl group, facilitating future construction of bioactive compounds.
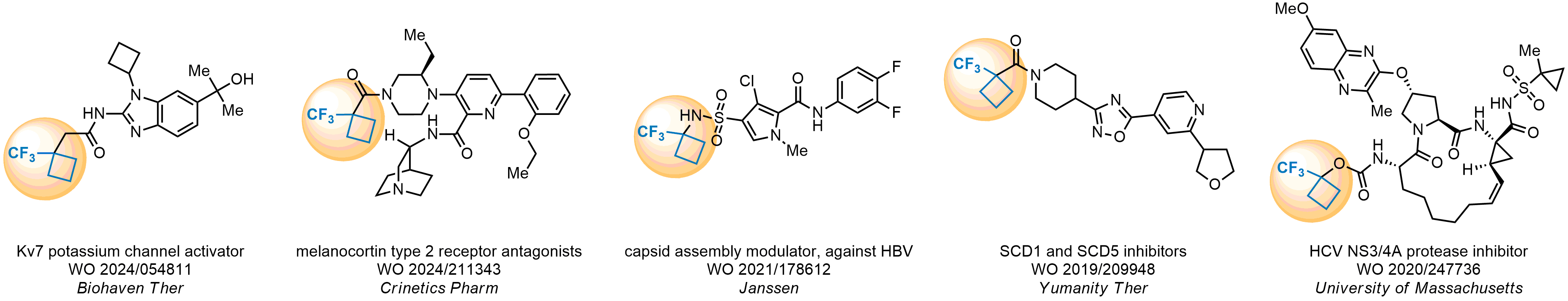
Key advantage
Download SD file
Download PDF file
We offer
Over 50 1-trifluoromethylcyclobutanes from stock on 5-10 gram scale.