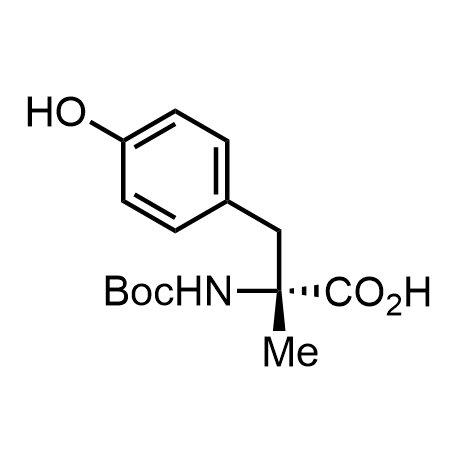
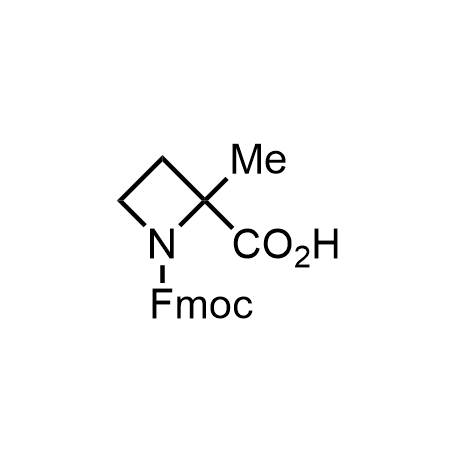
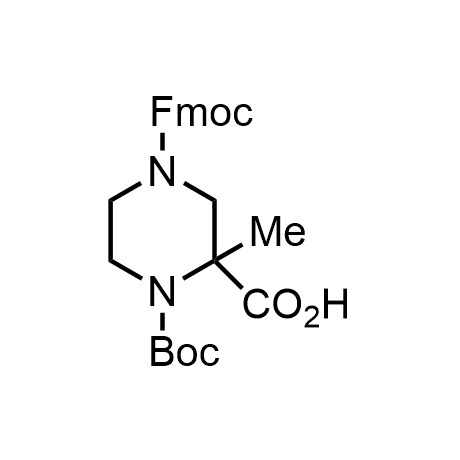
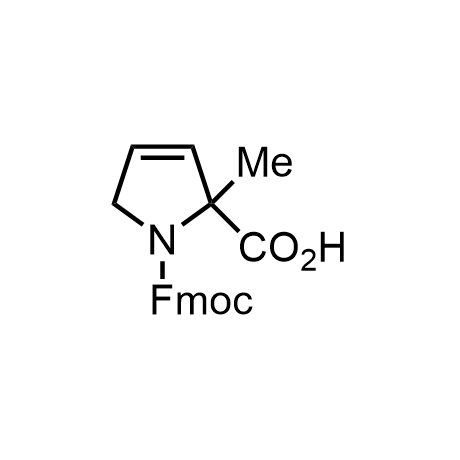
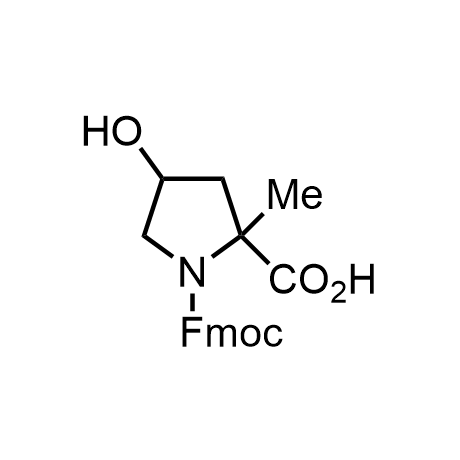
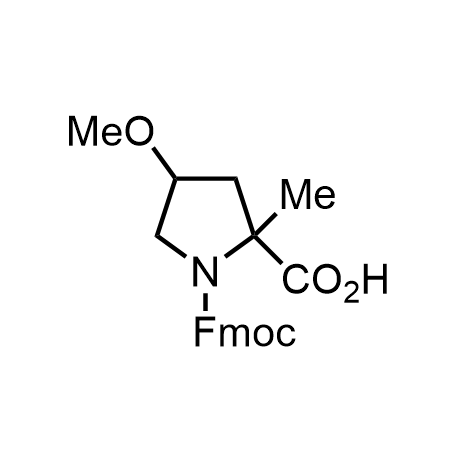
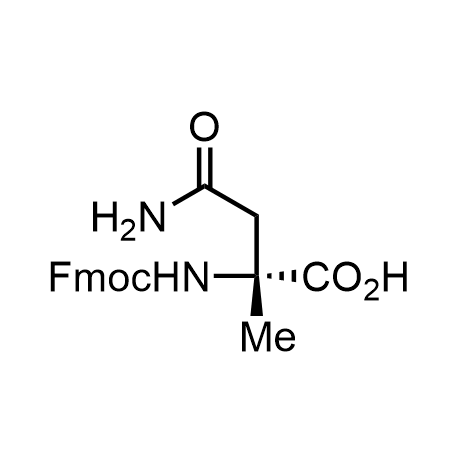
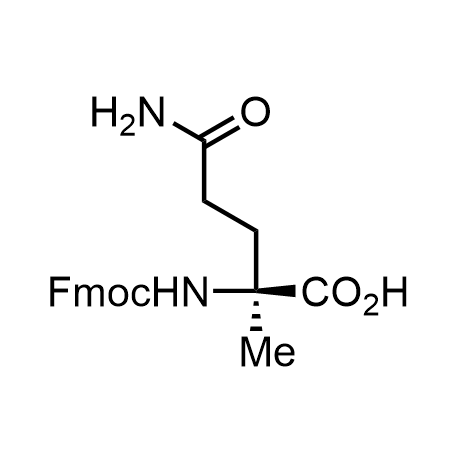
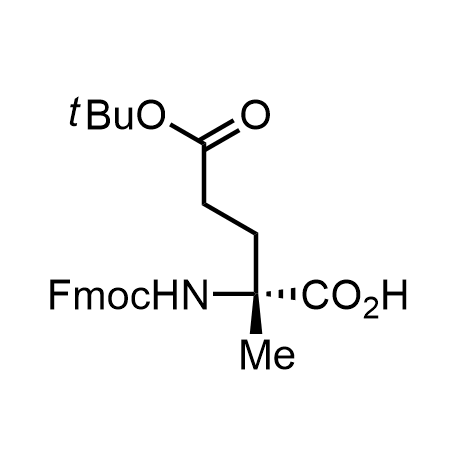
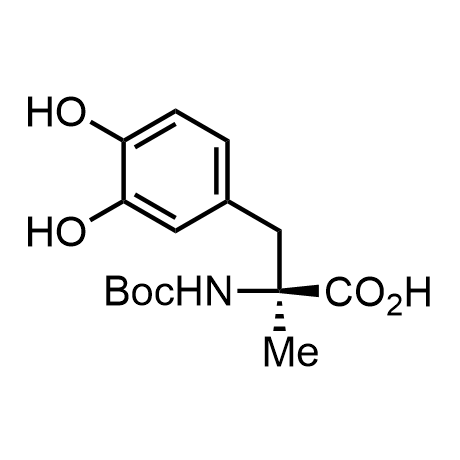
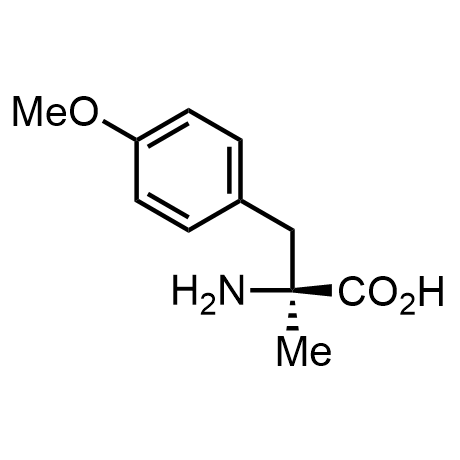
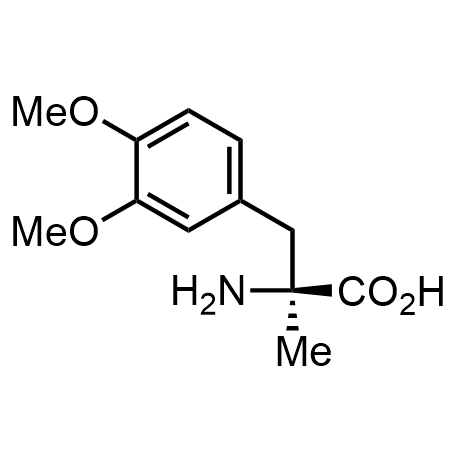
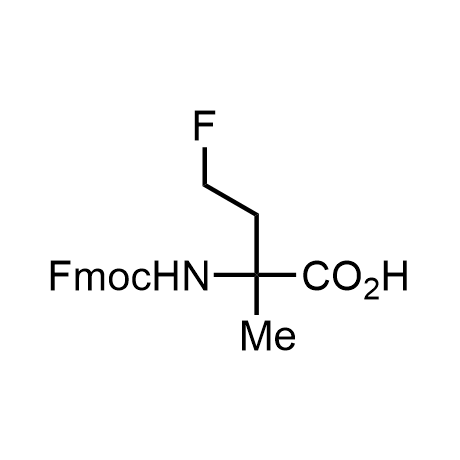
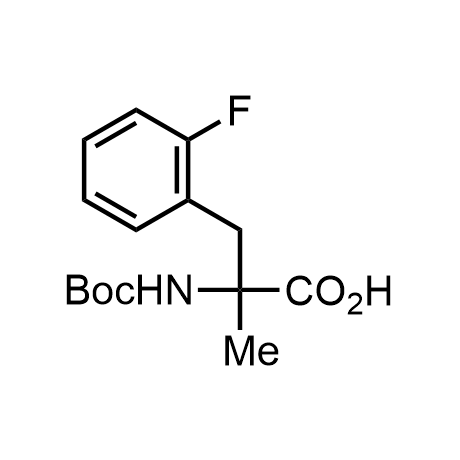
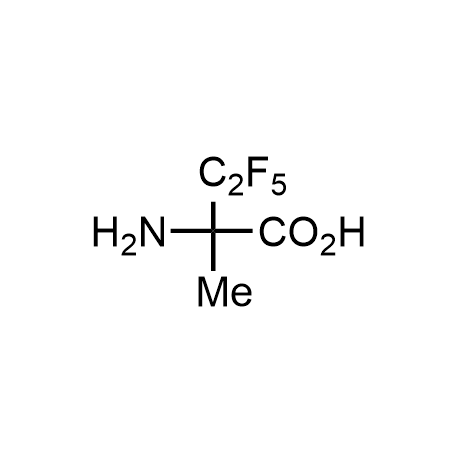
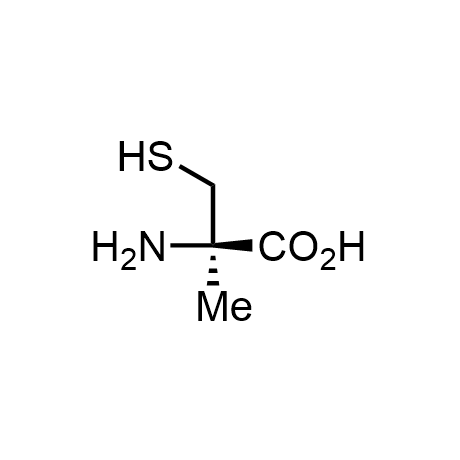
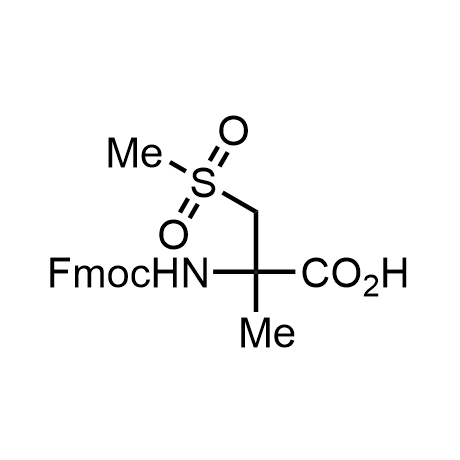
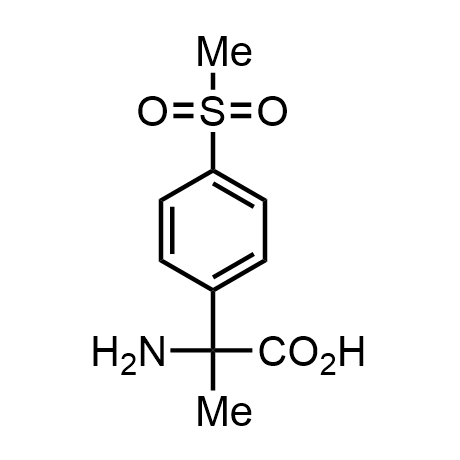
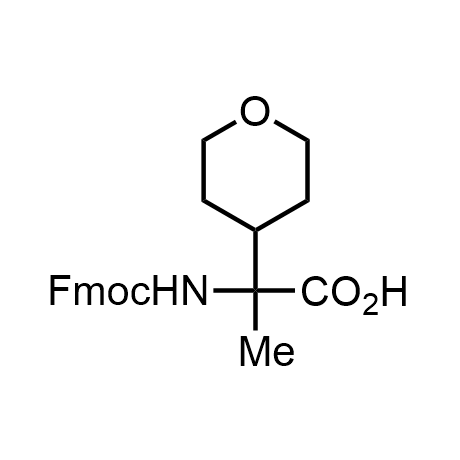
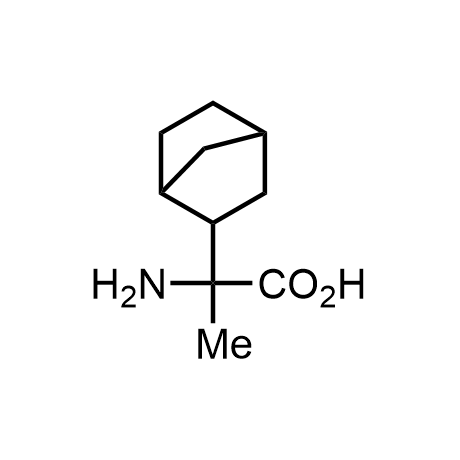
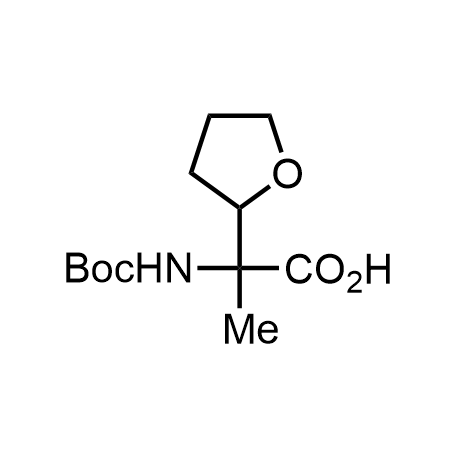
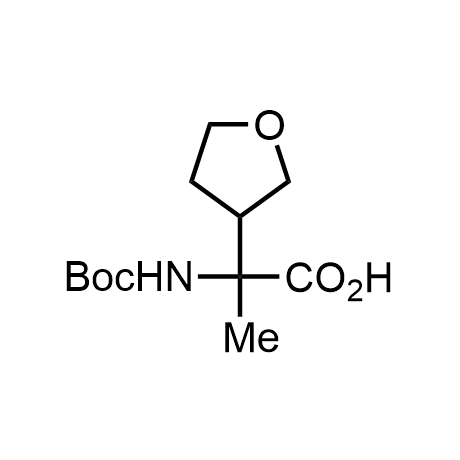
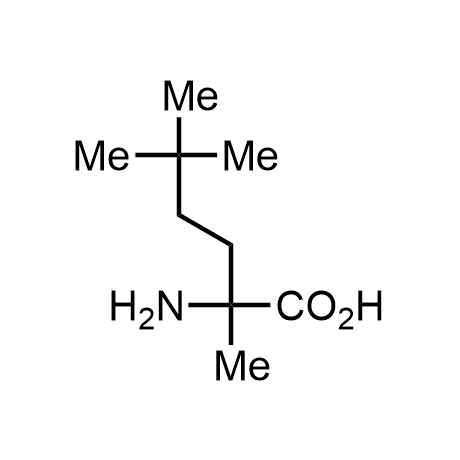
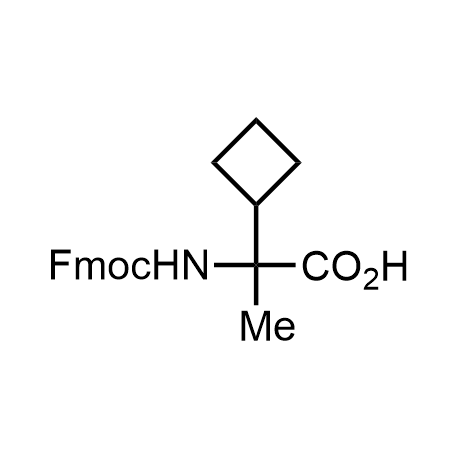
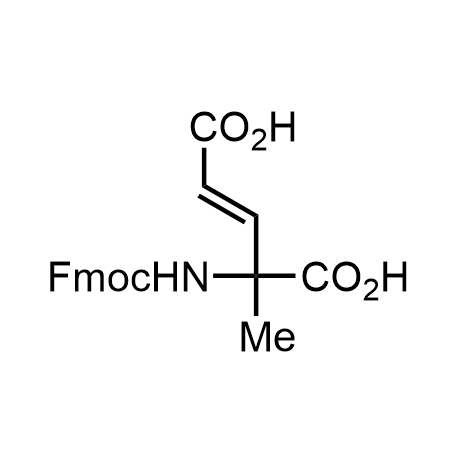
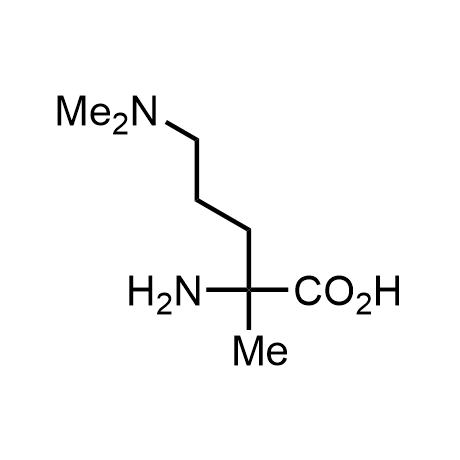
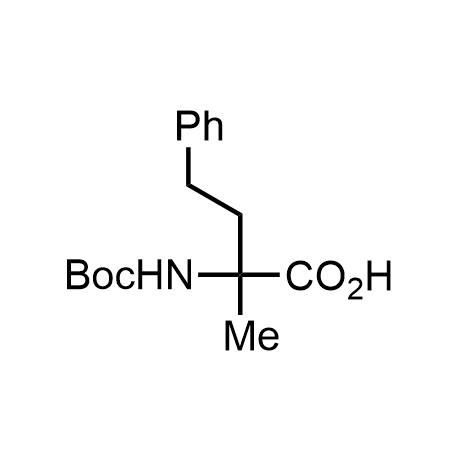
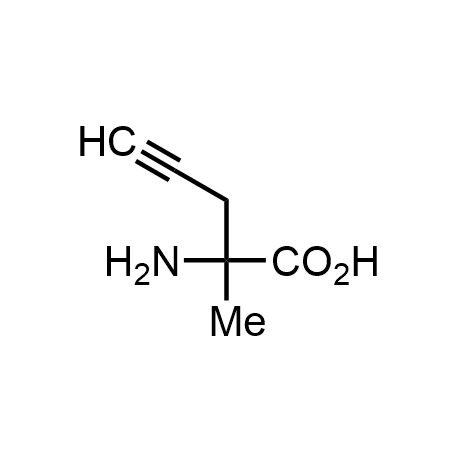
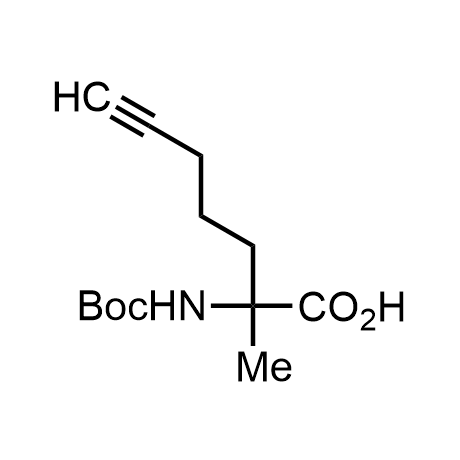
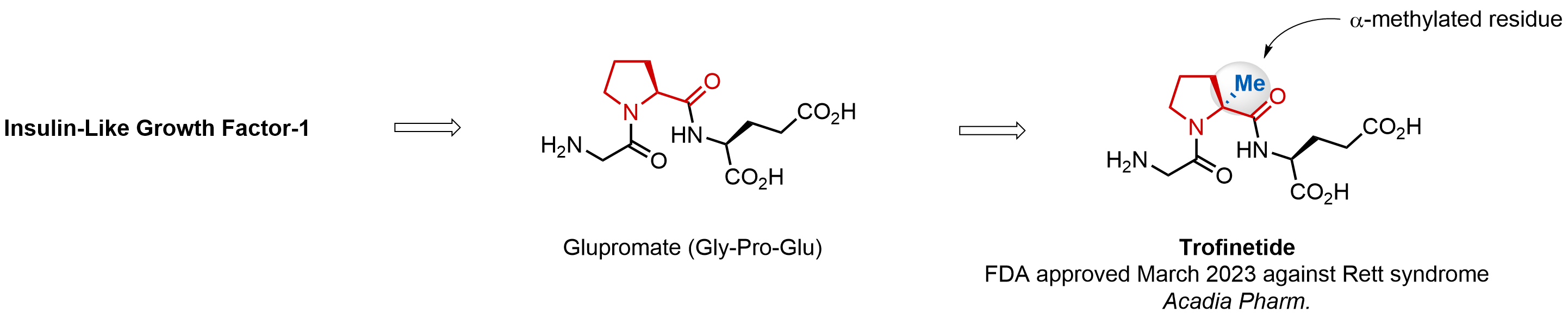
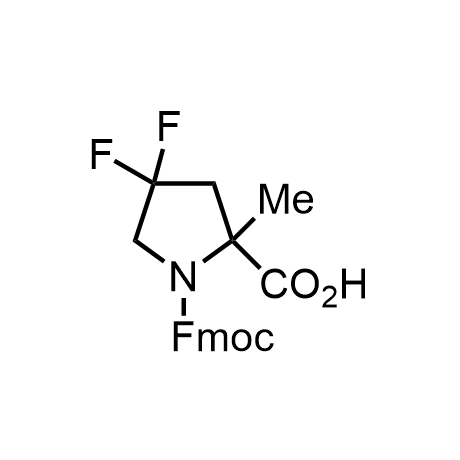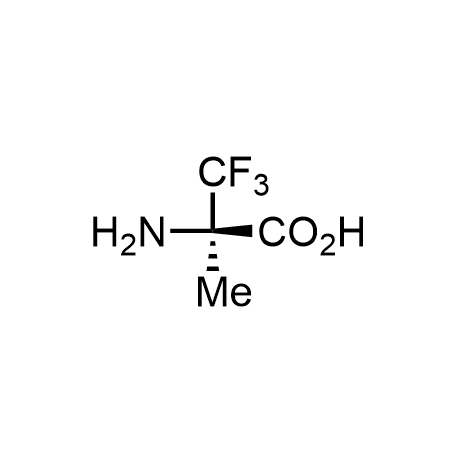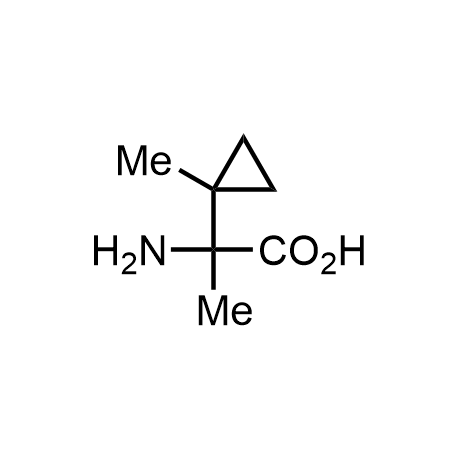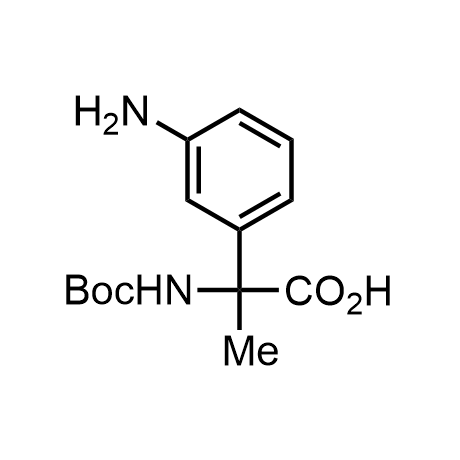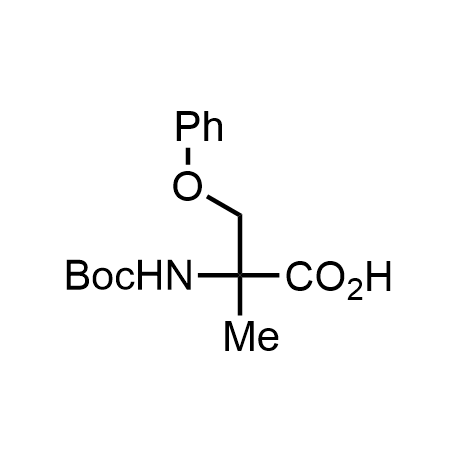α-Methylated amino acid residues help suppress peptide bond cleavage, reduce the conformational variability of peptides upon binding to their targets, and stabilize structures that would otherwise be labile due to α-proton abstraction. This modification is extremely useful in drug design. For example, trofinetide is a short neuroprotective peptide recently approved for the treatment of Rett syndrome. The molecule is a derivative of the short tripeptide Gly-Pro-Glu, developed by simple α-methylation of the central residue. Enamine offers a wide range of α-methylated amino acid structures for constructing bioactive peptides and peptidomimetics.
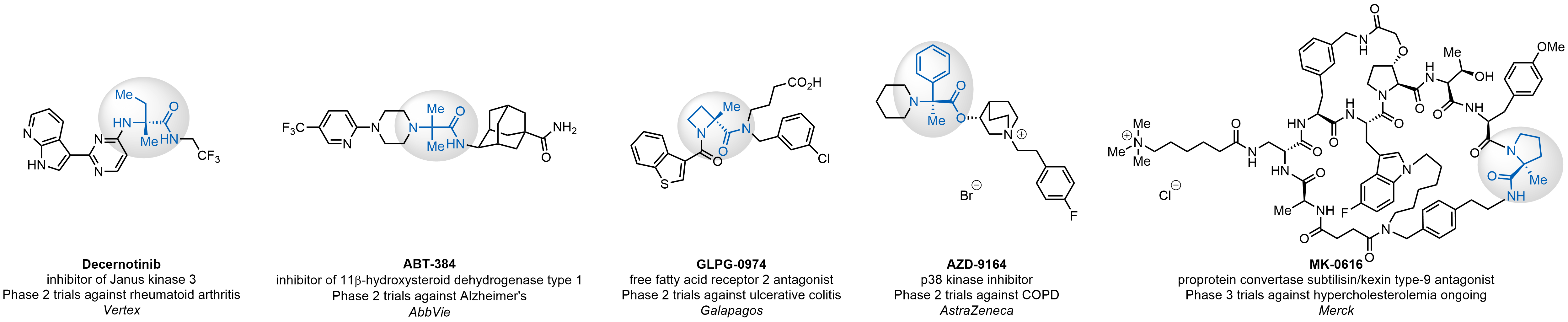
Case study
Download SD file
Download PDF file
We offer
Over 100 α-methyl amino acids from stock on 5-10 gram scale.