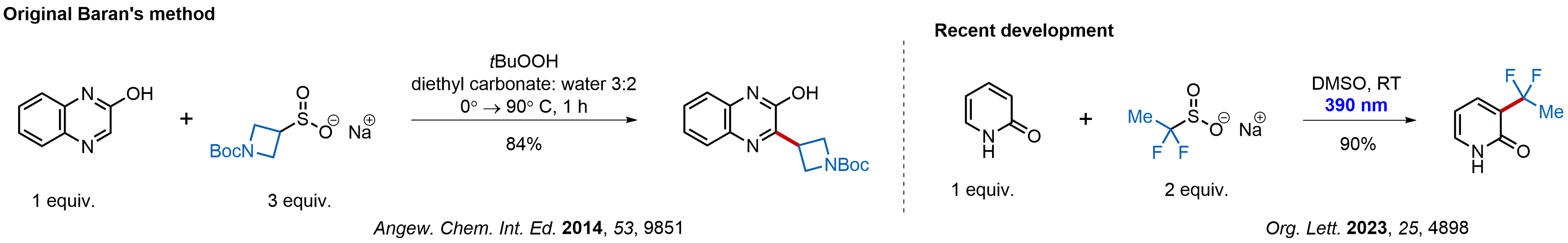
Nitrogenous heterocycles pervade the structures of drugs, although their desired substitution pattern is often hard to achieve. Since the original work from Baran group published in 2014, researches have been using alkyl sulfinates as versatile reagents for generating alkyl radicals with a great reactivity on nitrogen heterocycles. Such reactions require mild conditions owning to a relatively low energy of the C‑S bond. Resulting radicals can be directly incorporated into heterocyclic and related structures on both early and late stages of complex syntheses. Enamine chemists have mastered the synthesis of these advanced reagents and hoarded 91 alkyl sulfinates in our stock already.
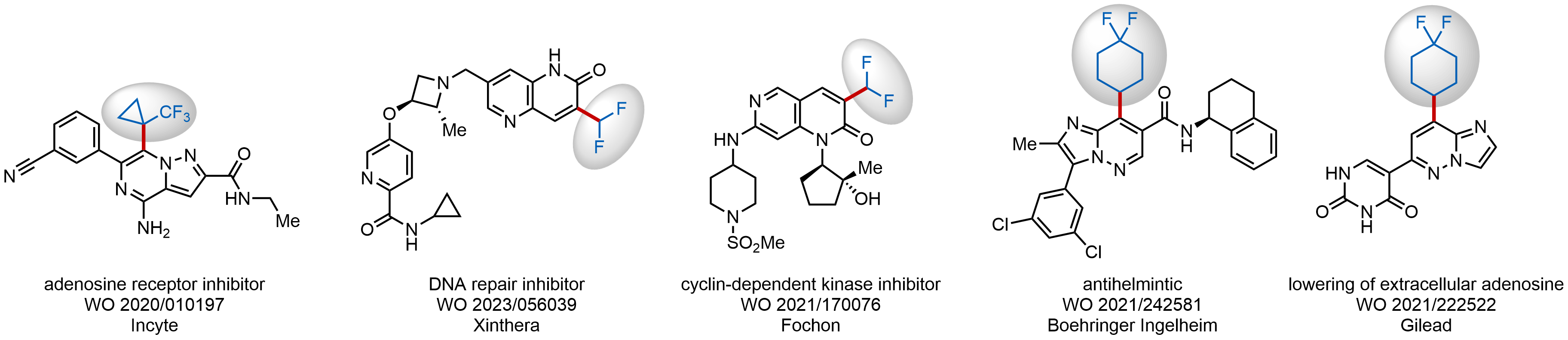
Examples
Download SD file
Download PDF file
We offer
Over 50 alkyl sulfinates from stock on 5-10 gram scale.



















































